Isolation and Partial Purification of Acidic Amylases from Manihot Esculenta, Crantz (Cassava) Peel
Panduranga Murthy G. , Sindhu K.S. , Mokshith M. C. , Surabhi Shrivastava, Lokesh S
1Centre for Shridevi Research Foundation (C.S.R.F),Department of Studies in Biotechnology,Shridevi Institute of Engineering & Technology (S.I.E.T),Sira Road,Tumkur-572 102,Karnataka,India
2Department of Studies in Biotechnology,University of Mysore,Mysore-570 006,Karnataka,India
Abstract
Manihot esculenta, Crantz (Cassava) peel was taken for the isolation of acidic amylases. The enzyme activity of 20% and 40% precipitate was found to be 0.02 and 0.18μmole/min, whereas, 50% precipitate did not show any enzyme activity. The fractionation of 40% precipitate on Sephadex G-75 column yielded two peaks with enzyme activity 0.02 and 0.08μmole/min respectively. The second peak indicated a rich source of amylase. A gradual increase in the enzyme activity was observed at 350C to 500C, along with drastic decrease at 500C onwards. A maximum activity was observed from 500C at pH 6.8 to 7.0. The activity of purified enzyme was 0.14μmole/min.Besides, substrate concentration between 1 to 3% which led to substantial increase in enzyme activity. Further, the enzyme activity of Cassava amylase against commercial starch was tested and found that, the enzyme activity increased with increase in time and remains constant after 80th min, whereas, on its own starch there was no activity till 30th min and after this time point, the activity gradually increases and it remains constant after 90 minutes. Thus, indicating Cassava acidic amylases act very rapidly on commercial starch than on its own starch.
Keywords
Cassava peel; Acidic amylases; Starch; Manihot esculenta; Crantz.
1. Introduction
The importance and development of Industrial biotechnology processing has lead to the utilization of enzymes from plant sources in various applications. One of these important enzymes is ‘amylase’,which hydrolyses starch to glucose. The vegetatively propagated woody plants such as ‘Cassava’ (Manihot esculenta,Crantz.) has received an increased attention as a favorable source for production of heterologous protein because of its ability to synthesis a vast amount of high value proteins and industrial enzyme i.e.,amylase [1-3]. Amylase belongs to the glycosyl hydrolase 13th family of enzymes,which is a large group of enzyme that act to hydrolyze the glycosidic bonds between two or more carbohydrate,or between a carbohydrate and a non-carbohydrate moiety. Amylases are produced during the germination of cereal grains,and this enzyme is key to the production of malt. Amylases have also found several valuable niches within our society,some of which are disease testing,fruit ripening,malt production,flour improvers etc [4-7].
Cassava,an important staple food crop in West Africa is processed into ‘gari’ and ‘fou fou’ in Nigeria. All the plant parts contain ‘cyanogenic glucosides’,with the leaves having the highest concentration. In the tuberous roots of Cassava,the peel has a higher concentration than the interior and the pulp has gain attention as a substrate for production of ethanol,due to containing relatively high portion of starch [5,8]. The various reports have been published on utilization of starch by fermentation with respect to the production of a wide range of extra-cellular enzymes [9-13]. However,there is little information available on the production of enzymes from plant sources. However,by keeping this fact,the study was initiated on the purification and characterization of amylase isolated from roots of Cassava peel with a view to further development of a amylase production using peel as a substrate and possible use in industries.
2. Materials and Methods
Cassava tubers were procured from authorized agricultural market,Mysore. All the Chemicals used in this experiment were of analytical grade obtained either from,Ranchem,Glaxo,Himedia etc. DNS,Bovine serum albumin (BSA),Sodium carbonate,Copper sulphate,Sodium potassium tartarate,Folin ciocalteau reagent,NaCl were from Merck,Sigma,SRL,and Ranbaxy. Other requirements are Sephadex G-75 column from commercial sources of reputed brand and Glass wares,plastic wares etc.
2.1 Preparation of Cassava peel extract
Whole tuberous roots of cassava were washed and peeled. Peeling was done in such a way that a thin white part of root was also taken. This peel was ground using distilled water and sieved. The liquid part was collected and centrifuged for 10 min at 3,000 rpm; the supernatant was stored in refrigerator for amylase analysis. This serves as crude.
2.2 Estimation of amylase activity from Cassava peel extract
Amylase activity was determined by DNS method. 1 ml of sample was added to 1 ml of standard starch solution (containing 1% soluble starch in 0.1 M phosphate buffer pH 7) and incubated at room temperature for 30 min. Reducing sugars were estimated by adding Di-nitrosalicylic acid (DNS) reagent,boiled for 5 min. and then cooled under running tap water. Then,2 ml of sterilized distilled water was added to dilute the solution. The absorbance of the resulting solution was determined at 540 nm with corning colorimeter against a reagent blank. One unit of amylase activity was taken as the amount of enzyme in 1 ml of crude amylase that produced 1 mg of reducing sugars under the standard assay conditions.
2.3 Isolation of starch from pulp of Cassava tubers
100 g of cassava tuber (without peel) was taken and it was grated. The grated pulp is again ground and sieved. The fiber collected in muslin cloth was washed twice or thrice. The sieved water was allowed to stand for 10 min. Then the supernatant was removed carefully and settled starch was dried at 35°C in hot air oven.
2.4 Determination of starch by Iodine Test
Two to three drops of iodine solution was added to 0.1 gm of powdered starch that was taken in a test tube,change in color reaction was observed. Further,the crude extract was taken from previously prepared sample and subjected for ammonium salt precipitation.
2.5 Salting out of protein from crude extract
100 ml of crude sample was taken and the proteins were salted out sequentially. For high molecular weight protein separation,20% salt was used and,15 g of finely powdered salt was dissolved slowly in crude sample. This was kept in refrigerator for minimum 6 hours at 40C. After 6 hours the solution was centrifuged at 5000 rpm for 10 min,at 40C. The pellet obtained was dissolved in 0.1M sodium chloride solution and refrigerated. For supernatant obtained after centrifugation was added with 15 g of salt for the separation of proteins of relatively small molecular weight. This forms 40% protein precipitation and further,steps are same as mentioned above. Similarly 50% precipitation was done and enzyme activity in each precipitation was analyzed.
2.6 Partial purification of amylase from crude sample of Cassava peel
The resin,Sephadex G-75 was washed twice with 0.1M sodium chloride. 1.5ml of sample (40% precipitation) was applied,and eluted by 0.1M sodium chloride.
2.7 Characterization of amylase enzyme isolated from Cassava peel
Effect of temperature on amylase activity
The effect of temperature was assayed at 5-80°C,pH 7 for 30 min. Each tube containing 1% starch and enzyme was incubated at temperatures,mentioned above. Amylase activity was determined for each temperature regime as earlier described earlier.
Effect of pH on amylase activity
The effect of pH on amylase activity was determined on starch solutions at pH 3 -10,30°C for 30 min. The amylase activity was determined as mentioned earlier.
Effect of substrate concentrations on amylase activity
Amylase activities of the 40% precipitation was determined at various substrate concentrations of 1-9% starch solutions containing 0.1 M phosphate buffer at pH 7 by the method described earlier.
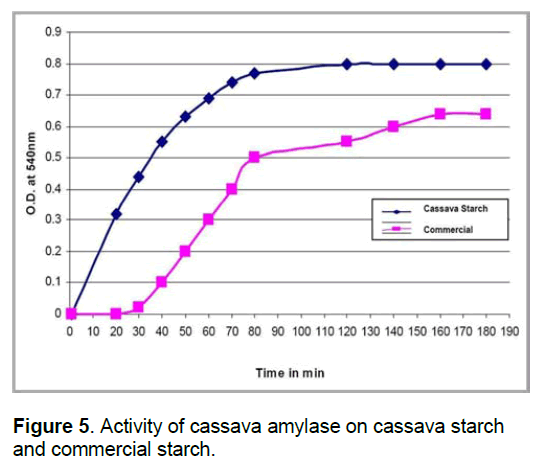
2.8 Activity of Cassava amylase on Cassava starch and commercial starch
10 ml of starch containing 0.1 M phosphate buffer of pH 7 and 10 ml of enzyme was incubated at room temperature. 2 ml of reaction mixture was taken at different time interval and reaction was arrested by adding 2N NaOH followed by boiling for 5 min. The activity was determined at different time interval from 0 -180 min randomly.
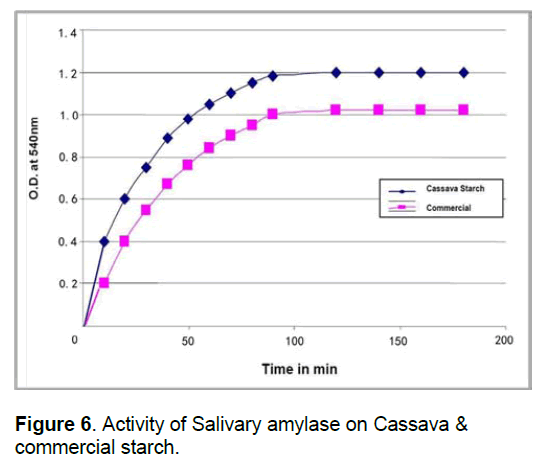
2.9 Activity of salivary amylase on Cassava starch and potato starch
Salivary amylase was diluted 200 times. Both cassava and potato starch are prepared separately in 0.1 M phosphate buffer of pH 7. Both starch solutions are mixed with enzyme and the activity was determined at different time intervals.
3. Results and Discussion
3.1 Isolation of amylase from Cassava peel
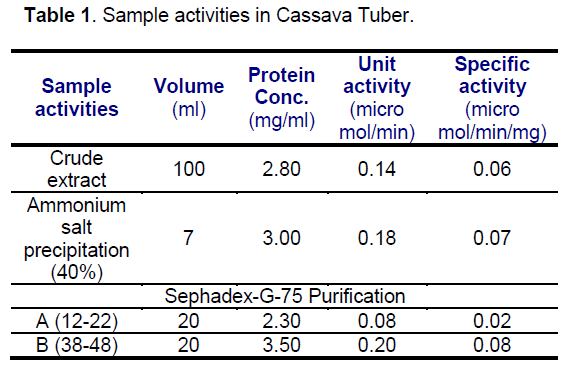
The squeezed out liquid from the cassava peel was assessed as a source of amylase. The result of the study indicates the presence of amylase activity in the peel. The activity and the specific activity of the amylase were 0.14 moles / min and 0.06 in mole / min/ mg respectively. This specific activity was higher than the specific activity reported previous researchers [7,14,15,16]. This indicates that,the activity of this enzyme is appreciably higher when compared to some plant amylases (Table 1).
The starch obtained after tuber processing is about 20%. This is amorphous,white material .The recovered starch when compared to other root tubers is high.
Two to three drops of Iodine solution was added to 0.1 gm of powdered starch that was taken in a test tube. The reaction mixtures showed blue color for the above test,which confirmed the presence of starch.
The activity of each precipitate dissolved in 0.1 M NaCl was used to determine its activity. The activities of 20%,40% and 50% precipitates were 0.02,0.18 and 0.00 micromole / min. This indicates that,the presence of amylase in 40% precipitate. And the specific activity of 40% was 0.05 u mol / min / mg of protein. For further characterization,40% precipitate was used as a source of amylase.
3.2 Partial purification of amylase from Cassava peel
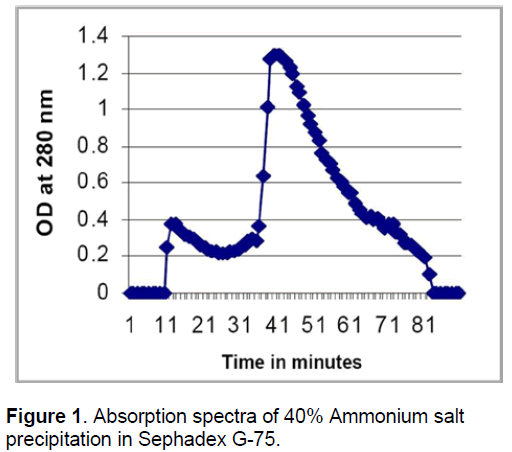
The elution profiles of the purified amylase from 40% precipitate was observed,two peaks [A (25-25),B (38-48)] having amylase activity were obtained after purification on sephadex G 75 gel. The specific activities of pooled peaks were 0.02 and 0.08μ mole/min./ mg of protein for peaks A & B respectively. This would possibly indicate that the amylase exists in the second peak (Figure 1).
3.3 Characterization of amylase enzyme
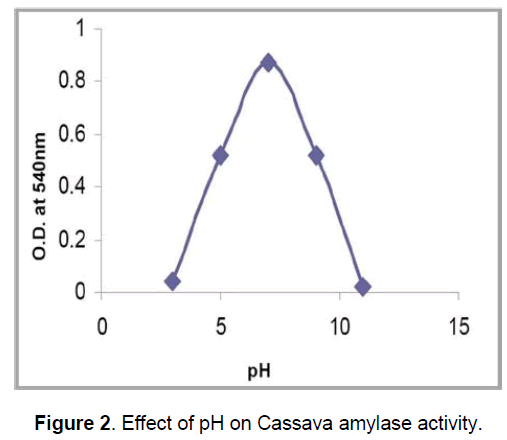
The effect of pH on the enzyme activity indicates that the amylase from cassava peel is active in the pH 6.8 – 7. This suggests that,the enzyme would be useful in processes that require pH range little acidic to neutral and vice versa. The amylase from certain fungus like A. flavus and M. Pusillus has activity at pH 6.0. The Beta amylases from Clostredium thermocellum SS8 also have an optimal activity at pH 6.0 [22,23]. However,there was drastic decline in the activity of the enzyme at pH above 7,which indicates that,the enzyme looses activity at alkaline region (Figure 2). However,the wastewater amylase showed some residual activity at pH 7-9,which,is in agreement with the earlier reports [4,9,17-19].
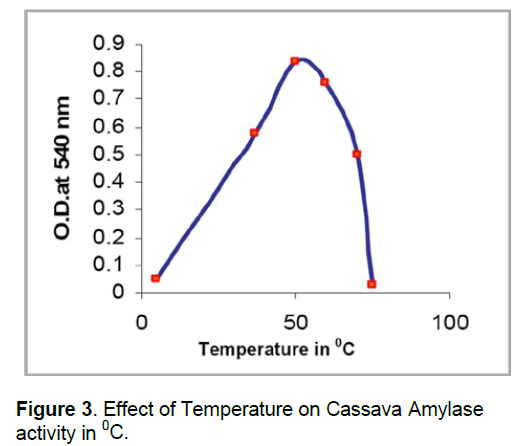
The effect of temperature on the activity of amylase indicated a gradual increase in the enzyme activity at 35-50°C while there was a drastic increase in the activity of the enzyme at the temperature 45-50°C. The activity of amylase was maximum at 50oC. However,the enzyme was active at 40-50°C. This attribute could be exploited in industrial activity that requires a sharp temperature (Figure 3).
The optimal temperature for maximum activity of the fermented cassava wastewater amylase is 60°C,which is same with that of Beta amylase from C. thermocellum SS8 [17,20,21]. The temperature 60°C is the optimal,temperature for A. flavus and M. pusillus [2,9] whereas 70°C is the optimal temperature for amylase activity from the A. niger. The main difference between these microbial and plant amylases is that,microbial amylase is active at wide temperature range from 40-60°C but this plant amylase does not show wide range but it is specific and sharp for particular temperature.
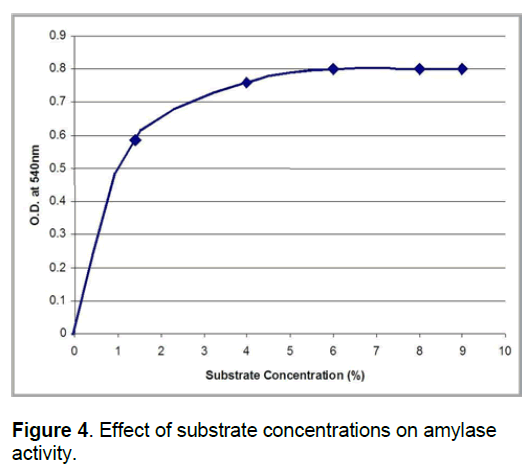
There was a drastic increase in the enzyme activity with increase in starch concentration from 1.0 to 3.0%,a minimal increase from 3.0 to 6.0% and subsequently the activity remained constant (Figure 4). This result is in agreement with earlier reports [4,5,9]. The report on the effect of substrate concentration on some fungal amylase activities,which indicated that increase in substrate concentration from 1 to 3% led to progressive increase in amylase activity (A. flavus,A. niger,R. oryzae and M. pusillus). This indicates that for optimal utilization of resources,the use of the amylase from this cassava amylase should be correlated with the starch to be hydrolyzed at 3% substrate concentration level.
The activity of cassava amylase on commercial starch increased with increase in time and remained constant after 80 min (Figure 5) whereas,on its own starch,there was no activity till half an hour. After 30 min. the activity gradually increases,it remains constant after one and half hour. This indicates that,the cassava amylase is acting very rapidly on commercial starch than on its own starch.
The activity of salivary amylase on cassava starch is more when compared to activity of salivary amylase on commercial starch. (Figure 6). This indicates that the salivary amylase is digesting cassava starch more easily than commercial starch.
4. Conclusions
The present study sought to assess,the potential utilization of Cassava peel to obtain the enzyme amylase. The amylase was purified and studied for its characterization. The ranges of pH,temperature and substrate concentrations were used to determine its optimum activity. The physico-chemical properties indicate the presence of amylase activity in cassava peel. The optimum pH,temperature and substrate concentration were determined as 7,50°C and 4% respectively.
The main difference between cassava amylase and fungal amylases known till now is that fungal amylases has wide temperature and pH whereas plant amylase i.e.,cassava amylase has specific temperature and pH for its activity. Since the amylases from fungal source have wide temperature and pH range activity,they are being used as industrial source of amylase.
The disadvantage of fungal amylases is that because of its wide pH and temperature range,it is difficult to stop its activity at desired level. If we go for plant amylase,it may not create a problem like above,since it has specific pH and temperature for its activity. Specially,it may not be a problem to obtain amylase from cassava source,because at a rough estimate,about 10 million tones of cassava are processed into ‘Garry’ annually in Nigeria alone [3,6,7,19,22,24].
Since these peels could make up to 10% of the wet weight of roots,they can constitute an important potential resource of industrial amylase. These cassava peels constitute as a major waste during processing of million tones of cassava into different products. After amylase extraction,these peels can be used as animal fodder [25,26,27,28].
From experimental studies,it is clear that cassava amylase show lower activity on its own starch but considerably higher activity on commercial starch. Interestingly,salivary amylase is more active on cassava starch than commercial starch. One of the other important facts to be considered is that almost all tuberous,starchy roots have both enzyme and substrate present together in the same part. But,its own enzyme does not show maximal activity on its own starch. However strange it may sound but it is true. Which molecules are regulating the activity of its own amylase is yet unclear.
Acknowledgements
The authors express sincere gratitude to the authorities of JSS College,Mysore for the initiation of the present research work and Principal,Shridevi Institute of Engineering & Technology (S.I.E.T),Department of Studies in Biotechnology & Engineering,Tumkur - 572 106 for providing facilities to complete the research work.
References
- Rocha G., Moore P., Amante L.R.C.E.R. (2005) Cassava and Corn starch in Maltodextrin production. Quim. Nova, 28: 596-600.
- Hyun H.H., Zeikus J.G. (1985) General Biochemical Characterisation of Thermostable extracellular ß-amylase from Clostridium thermosulfurogenes. Appl. and Environ. Microbiol., 49: 1162-1167.
- Jyothi A.N., Moorthy S.N., Rajasekharan K.N. (2007) Studies on the synthesis and properties of hydroxypropyl derivatives of cassava (Manihot esculenta Crantz) starch. J. Sci. Food. Agric., 87: 1964-1972.
- Reungsang A., Imai T., Chaiprasert P. (2004) “Biohydrogen production from Cassava starch Manufacturing Waste water” ,Khon Kaen University,Khon Kaen,Thailand.The Joint International Conference on “Sustainable Energy and Environment (SEE)”, Hua Hin, Thailand.
- Chang C.T., Liou H.Y., Tang H.L., Sung H.Y. (1996) Activation, purification and properties of ß- amylase from sweet potatoes (Ipomea batatas), Biotechnol. Appl. Biochem., 24: 113–118.
- Jyothi A.N., Sasi Kiran K., Wilson B., Moorthy S.N., Bala N. (2007) Wet storage of cassava starch: Use of sodium metabisulphite and acetic acid and the effect on starch properties. Starch/Stärke, 59: 141-148.
- Voet D., Voet G.J., Pratt, C.W. (2006) “Starch and Amylase”. In Fundamentals of Biochemistry, Johnwiley and Sons (Asia) Pvt. Ltd, pp. 756.
- Cardoso A.P., Mirione E., Ernesto M. (2005) Processing of cassava roots to remove cyanogens. Journal of Food Composition and Analysis, 18: 451-460.
- Alli A., Ogbonna C.I.C., Rahman A.T.M.F. (1998) Hydrolysis of certain Nigerian Cereal starch using crude fungal amylase, Nig. J. Biotechnol., 9: 24-36.
- Oboh G. (2005) Isolation and Characterization of amylase from fermented Cassava (Manihot esculenta Crantz) waste water. African journal of Bio-technology, 4: 1117-1123.
- Rocha G., Moore P., Amante L.R.C.E.R. (2005) Cassava and Corn starch in Maltodextrin production. Quim. Nova, 28: 596-600.
- Ilori M.O., Amund O.O., Omidui O. (1997) Purification and properties of an alpha amylase produced by a Cassava fermenting strain of Micrococcus luteus. Folia Microbiol., 42: 445-449.
- Nkere C.K., Mbanaso E.N.A. (2009) In Vitro Culture of Cassava (Manihot esculenta Crantz): Assessment of Cassava Starch from Different Varieties as Gelling Agent in Culture Medium. International Journal of Applied Agricultural Research, 4(3): 0974-4754.
- Oboh G. (2006) Nutrient enrichment of Cassava peels using a mixed culture of Saccharomyces cerevisae and Lactobacillus spp solid media fermentation techniques. Elect. J. Biotechnol., 9(1): 46-49.
- Oboh G., Ajele J.O. (1997) Effects of some metallic chlorides on the activity of ß-amylase from sweet potatoes.Nigerian J. Biochem. Mol. Biol., 12: 73–75.
- Oboh G., Akindahunsi A.A. (2003) Biochemical changes in Cassava products (flour and garri) subjected to Saccharomyces cerevisae solid media fermentation. Food Chem., 82: 599-602
- Oboh G., Akindahunsi A.A., Oshodi A.A. (2002) Nutrient and anti-nutrient content of Aspergillus niger fermented cassava products (flour and gari). J. Food Composition and Anal., 15: 617-622.
- Oke O.L. (1968) Cassava as food in Nigeria. World Rev. Nutr. Diet., 9: 227-250.
- Wingertzahn M.A., Teichberg S., Wapnir R.A. (1999) Modified starch enhances absorption and accelerates recovery in experimental diarrhea in rats. Pediatr Res., 45: 397-402.
- Hyun H.H., Zeikus J.G. (1985) General Biochemical Characterisation of Thermostable extracellular ß-amylase from Clostridium thermosulfurogenes. Appl. and Environ. Microbiol., 49: 1162-1167.
- Swamy M.V., Sairam N., Seenayya G. (1994) ß-amylase from Clostridium thermocelluum SS8 a thermophillic, anaerobic, cellulolytic bacterium. Lett. Appl. Microbiol., 18: 301-304.
- Jyothi A.N., Moorthy S.N., Rajasekharan K.N. (2006) Synthesis and characterization of dodecenyl derivatives of cassava starch. Journal of Root Crops, 32: 107-114.
- Jojo V.V., Rajesh R.R., Pillay V.V. (2007) Identification of active principles of manihot esculenta and cerbera odallum by thin layer chromatography: The potential for misinterpretation in forensic cases.Journal of the Indian Society of Toxicology, 3(1): 0973-3558.
- Wapnir R.A., Wingertzahn M.A., Moyse J., Teichberg S. (1998) Proabsorptive effects of modified tapioca starch as an additive of oral rehydration solutions. J Pediatr Gastroenterol Nutr., 27: 17-22.
- Akindahunsi A.A., Oboh G., Oshodi A.A. (1999) Effect of fermenting cassava with Rhizopus oryzae on the chemical composition of its flour and gari. La Rivista Italiana Delle Sostanze Grasse, 76: 437-440.
- Reungsang A., Sansyoka S., Imai T., Chaiprasert P. (2004) Biohydrogen production from Cassava starch Manufacturing Wastewater. In The Joint International Conference on “Sustainable Energy and Environment (SEE)”, Hua Hin, Thailand, 3-036(O): 319-327.
- Oboh G. (2005) Isolation and Characterization of amylase from fermented Cassava (Manihot esculenta Crantz) waste water. Afr. J. of Biotechnology, 4: 1117–1123.
- Rocha G., Moore P., Amante L.R.C.E.R. (2005) Cassava and Corn starch in Maltodextrin production. Quim. Nova, 28: 596-600.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences