Hot-PBS Extract of Vibrio vulnificus Induces NF-ÃÆà ½ÃâúB Activation
Noboru Nakasone, Yasunori Ogura, Naomi Higa, Claudia Toma, Yukiko Koizumi, Toshihiko Suzuki, Tetsu Yamashiro
1Department of Bacteriology, Graduate School of Medicine, University of the Ryukyus, Nishihara, Japan
2Department of Food Science and Nutrition, Nara Women's University, Japan
3Department of Pathology and Comprehensive Cancer Center, University of Michigan Medical School, USA
4Department of Bacterial Pathogenesis, Infection and Host Response Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Japan
Received date: March 31, 2017; Accepted date: April 18, 2017; Published date: April 25, 2017
Citation: Nakasone N, Ogura Y, Higa N, et al. Hot-PBS Extract of Vibrio vulnificus Induces NF-κB Activation. Electronic J Biol, 13:2
Abstract
Background: Vibrio vulnificus causes necrotizing fasciitis and liver dysfunction. Although many studies have attempted to clarify the molecular mechanism by which V. vulnificus generates these symptoms, the mechanism remains poorly understood. Methods and findings: We focused on determining the factor derived from V. vulnificus that induces inflammation. We found that an extract of V. vulnificus prepared using hot phosphate buffered saline (PBS) markedly induced the activation of NF-κB. The activation, however, was not caused by lipopolysaccharides, the capsule, peptidoglycans, or NOD1/NOD2 ligands, all of which are known to activate NF-κB. Conclusion: A factor in a hot-PBS extract from V. vulnificus might activate NF-κB through an unknown receptor, and such a factor might play an important role in the etiology of diseases caused by V. vulnificus.
https://sporbahisleri.livejournal.com https://wakelet.com/@SporBahisleri67459 https://theomnibuzz.com/author/sporbahisleri/ https://lessons.drawspace.com/profile/322207/sporbahisleri/workflow https://writeupcafe.com/profile/sporbahisleri/ http://www.pearltrees.com/sporbahisleri https://pharmahub.org/members/26874/blog https://www.zupyak.com/u/Spor-Bahisleri/ https://www.metroflog.co/sporbahisleri https://www.fuzia.com/fz/spor-bahisleri https://tr.pinterest.com/sporbahislerim/ https://my.getjealous.com/sporbahisleri https://sporbahisleri.contently.com https://hubpages.com/@sporbahisleri https://www.tumblr.com/sporbahisleri https://hub.docker.com/u/sporbahislerim https://betsiteleri.blogfree.net https://betsiteleri.amebaownd.com https://sporbahisleri.pixnet.net/blog https://betsiteleri.seesaa.net https://betsiteleri.threadless.com https://betsiteleri.neocities.org https://bahissiteleri.localinfo.jp https://betsiteleri.shopinfo.jp https://teletype.in/@betsiteleri https://ubl.xml.org/users/sporbahisleri https://betsiteleri.educatorpages.com https://betsiteleri.onlc.fr https://sporbahisleri.gumroad.com
Keywords
V. vulnificus; Hot-PBS extract; Inflammation; NF-κB.
1. Introduction
Vibrio vulnificus is a gram-negative, curved, rod-shaped halophilic bacterium that causes severe wound infections and septicemia and is frequently fatal in hosts with liver disease or immunosuppression [1,2]. Septic shock, which occurs in fatal cases, has been attributed to a variety of virulence factors of V. vulnificus, such as bacterial Lipopolysaccharide (LPS), Capsular Polysaccharides (CPS), Hemolysin (VvhA) and Multifunctional Autoprocessing RTX Toxin (MARTX) [3-7]. However, the role of these factors in the local inflammation caused by V. vulnificus remains unclear [1-13].
NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a transcription factor that plays a central role in immune responses and is involved in numerous physiological processes, such as acute and chronic inflammation, cell proliferation, and apoptosis. In this study, we found that a hot-PBS extract from V. vulnificus markedly induced NF-kB activation, and we examined the role of this hot-PBS extract in NF-kB activation.
2. Materials and Methods
2.1 Bacterial and culture conditions
The wild-type V. vulnificus MO6-24/O strain was provided by Dr. Antia C. Wright (University of Florida, Gainesville, FL). To eliminate the effects of hemolysin and MARTX toxin, double mutants of V. vulnificus (DvvhA/DrtxA) were used. The isogenic V. vulnificus double mutants (DvvhA/DrtxA) were provided by Dr. T. Iida (Osaka University, Japan). The bacteria were cultured in 100 ml of terrific broth at 37°C for 13 h, with shaking.
2.2 Preparation of bacterial extract
Bacteria were harvested by centrifugation at 10,000 xg for 10 min, washed in phosphate buffer saline (PBS), resuspended in 10 ml of PBS, and then heated at 65°C for 1 h. After centrifugation at 15,000 xg for 10 min, the supernatant was filtered through a 0.25 mm membrane filter. The filtrate was then stored at -80°C.
2.3 NF-kB activity
A reporter plasmid that expresses luciferase depending on the NF-kB activity level (pBVI-Luc) was transfected into the HEK293T cells using a control reporter plasmid that expresses b-galactosidase constitutively under the control of the promoter of elongation factor 1 (pEF-BOS-b-Gal) using the calcium phosphate method. Eight hours after transfection, the cells were treated with the bacterial extract, and 16 hours after exposure to the extract, the cells were lysed. The luciferase activity in the cell lysate was measured using a light emission assay with a commercially available kit (Promega, WI) and the b-galactosidase activity was measured using a coloring assay with ortho-nitrophenyl-b-galactoside. To evaluate the relative activity of NF-kB in the cells, the luciferase activity was divided by the activity of b-galactosidase. To test the ability of bacterial extract to activate NF-kB through NOD1 or NOD2, pMX-HA-NOD1 or pMX-HA-NOD2, respectively, was transfected into HEK293T cells with the luciferase and b-galactosidase reporter plasmids and evaluate the NF-kB activityas described above [8].
2.4 Preparation of LPS, CPS and peptidoglycan
Lipopolysaccharide (LPS), Capsular Polysaccharide (CPS), and peptidoglycan were prepared according to the methods described by Westphal and Lüderitz [9], Reddy et al. [10] and Gan et al. [11], respectively. Each extract was subjected to periodic acid-Schiff staining.
2.5 Enzyme treatment
The hot-PBS extracts were treated with protease K, DNase I, lysozyme and lipase at a concentration of 100 mg/ml each at 37°C for 1 h and the resulting abilities of the extracts to induce NF-kB were examined.
2.6 Ultrafiltration and anion-change column chromatogram
Ultrafiltration was performed using commercially available centrifugal filter devices (Merck Millipore, MA). Gel filtration and anion exchange column chromatography were performed using the Sephadex G25 (Sigma Aldrich, MO) (1 kDa-5 kDa cut-off) (150 mm × 10 mm) and the Q-Sepharose Fast Flow (GE Healthcare, IL) (100 mm × 15 mm), respectively.
3. Results
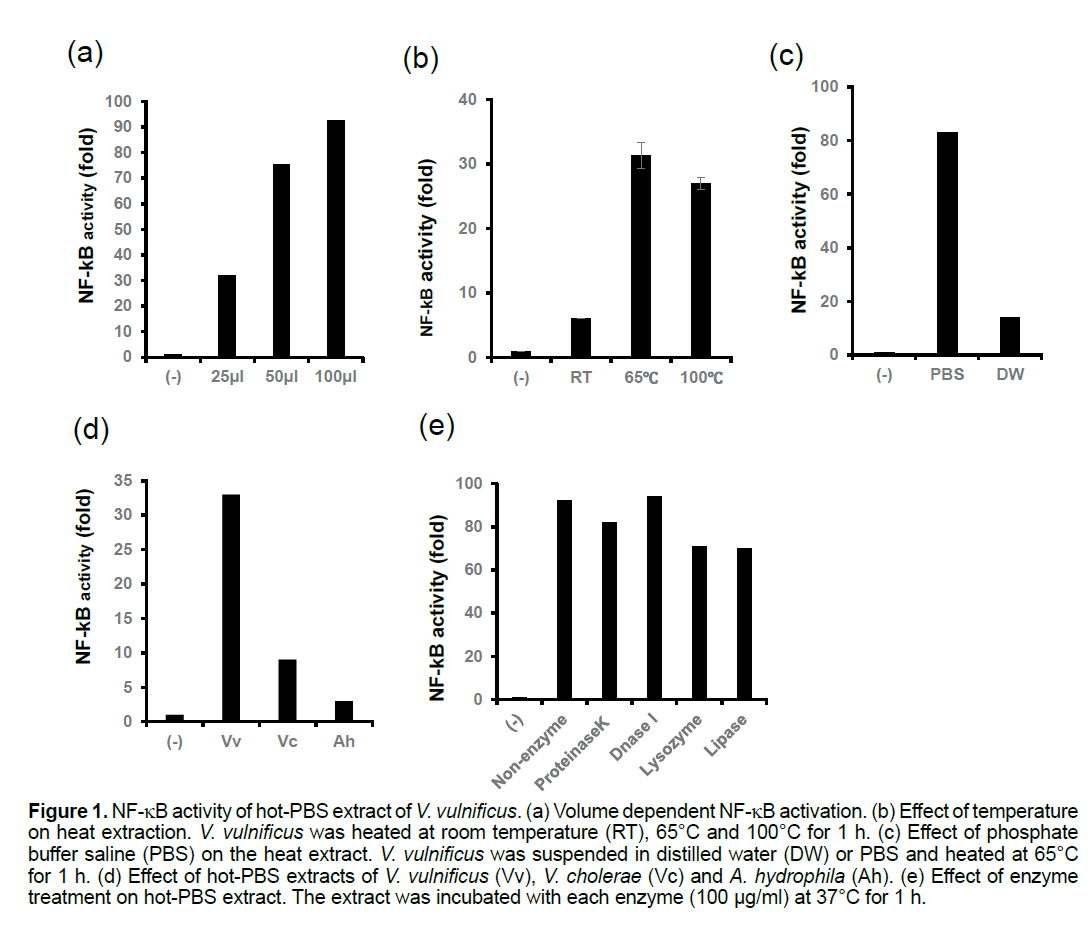
We found that hot-PBS extract from V. vulnificus activates NF-kB in HEK293T cells in a dose-dependent manner (Figure 1a). For the full activation of NF-kB, heat treatment of the bacterial suspension was required (Figure 1b), and the bacteria had to be suspended in PBS instead of pure water (Figure 1c). The hot-PBS extract of V. vulnificus induced a stronger activation of NF-kB than those of Vibrio cholera or Aeromonas hydrophila (Figure 1d). The ability of the hot-PBS extract to induce NF-kB was not affected by treatment with protease K, DNase I, lysozyme or lipase (Figure 1e).
Figure 1:NF-kB activity of hot-PBS extract of V. vulnificus. (a) Volume dependent NF-kB activation. (b) Effect of temperature on heat extraction. V. vulnificus was heated at room temperature (RT), 65°C and 100°C for 1 h. (c) Effect of phosphate buffer saline (PBS) on the heat extract. V. vulnificus was suspended in distilled water (DW) or PBS and heated at 65°C for 1 h. (d) Effect of hot-PBS extracts of V. vulnificus (Vv), V. cholerae (Vc) and A. hydrophila (Ah). (e) Effect of enzyme treatment on hot-PBS extract. The extract was incubated with each enzyme (100 mg/ml) at 37°C for 1 h.
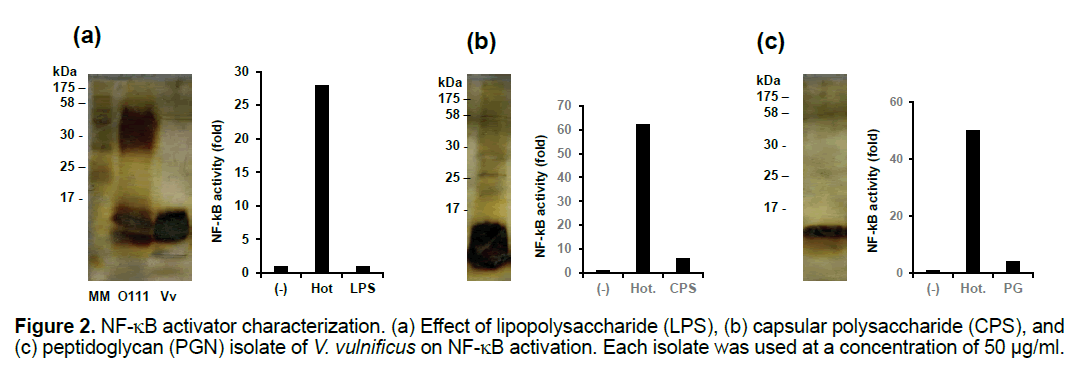
We next examined whether LPS, CPS, or peptidoglycan prepared from V. vulnificus was capable of activating NF-kB to the same extent as the hot-PBS extract. Even though 5 mg of these compounds were used to stimulate the HEK293T cells, none of these compounds activated NF-kB to the same extent as the hot-PBS extract (Figures 2a-2c).
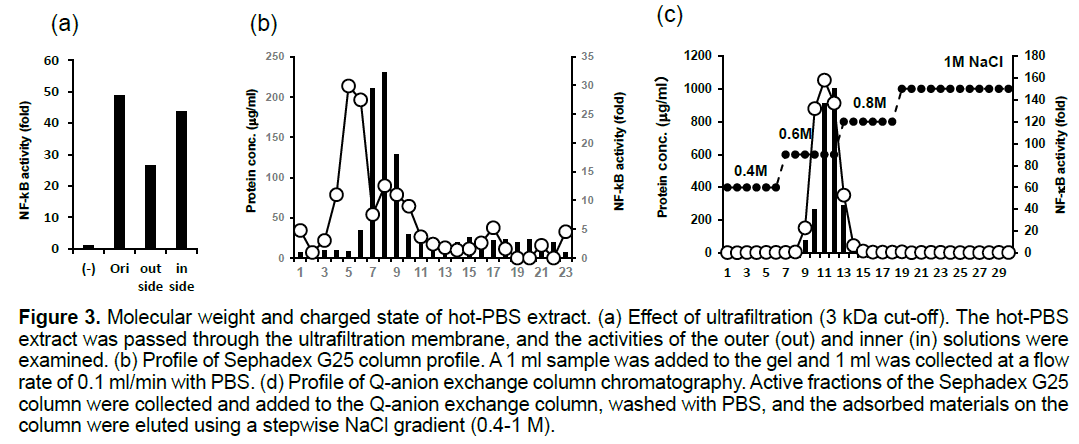
To estimate the molecular weight of the factor responsible for the NF-kB activation, we subjected the extract to size fractionation using ultrafiltration. As the activity was retained after passage through a 3kDa cut-off membrane (Figure 3a), the molecular weight of the NF-kB activator was estimated to be less than 3 KDa.
Gel filtration column chromatography using Sephadex G-25 (Figure 3b) showed that the size of the molecule responsible for the NF-kB activation was 3 kDa or smaller, which was similar to the size estimated using ultrafiltration.
Next, we tested whether the molecule could be fractionated using anion exchange column chromatography using Q-Sepharose Fast Flow anion exchange resin. As a result, the NF-kB activating factor was shown to bind to the column in PBS and to be eluted by a phosphate buffer containing 0.6 M NaCl (Figure 3c).
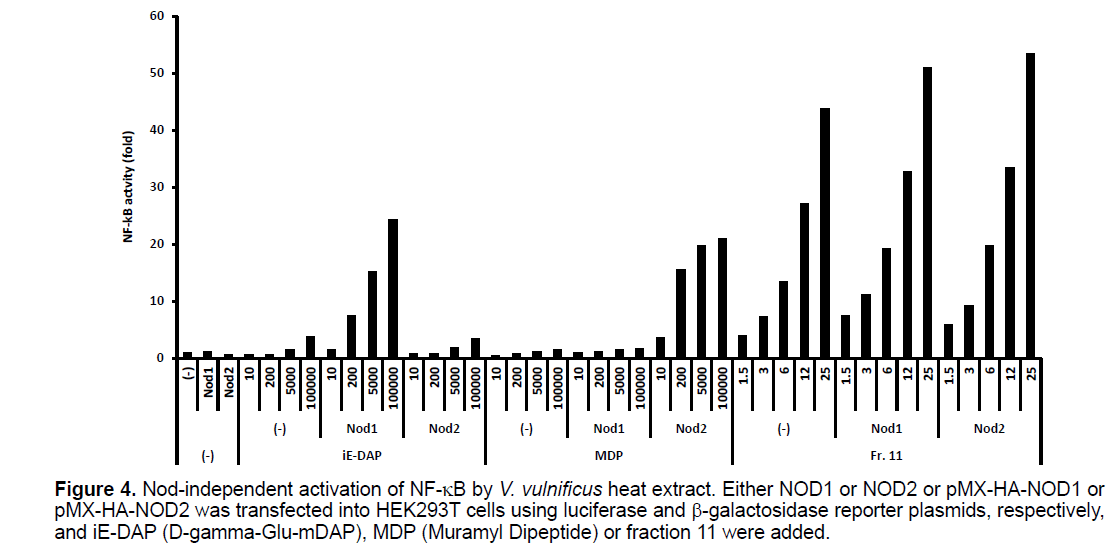
Since HEK293T cells are considered to express a certain amount of NOD1 and NOD2 protein, the V. vulnificus extract might activate NF-kB through endogenous NOD1 or NOD2 in HEK293T cells. Consequently, we examined whether the V. vulnificus extract activates NOD1 or NOD2. As shown in Figure 4, 100000 ng/ml of iE-DAP (NOD1 ligand) or MDP (NOD2 ligand) induced about a 4-fold activation of NF-kB in the plain HEK293T cells, while the same concentration of iE-DAP or MDP induced a more than 20-fold activation in the HEK283T cells that are forced to express NOD1 or NOD2, respectively. As 1.5 ml of fraction 11 induced about a 4-fold activation in the plain HEK293T cells, if the activation is mediated by NOD1 or NOD2, then the same amount of fraction 11 should induce a 20-fold activation in HEK293T cells expressing NOD1 or NOD2, respectively. However, 1.5 ml of fraction 11 induced only a 7-fold and a 6-fold activation of NF-kB in cells expressing NOD1 and NOD2, respectively. Thus, NF-kB activation by fraction 11 in HEK293T cells cannot be explained by endogenous NOD1 and NOD2 alone.
Figure 3. Molecular weight and charged state of hot-PBS extract. (a) Effect of ultrafiltration (3 kDa cut-off). The hot-PBS extract was passed through the ultrafiltration membrane, and the activities of the outer (out) and inner (in) solutions were examined. (b) Profile of Sephadex G25 column profile. A 1 ml sample was added to the gel and 1 ml was collected at a flow rate of 0.1 ml/min with PBS. (d) Profile of Q-anion exchange column chromatography. Active fractions of the Sephadex G25 column were collected and added to the Q-anion exchange column, washed with PBS, and the adsorbed materials on the column were eluted using a stepwise NaCl gradient (0.4-1 M).
Figure 4. Nod-independent activation of NF-B by V. vulnificus heat extract. Either NOD1 or NOD2 or pMX-HA-NOD1 or pMX-HA-NOD2 was transfected into HEK293T cells using luciferase and -galactosidase reporter plasmids, respectively, and iE-DAP (D-gamma-Glu-mDAP), MDP (Muramyl Dipeptide) or fraction 11 were added.
4. Discussion and Conclusion
NF-kB is activated in response to various stresses and stimuli received from the surrounding environment and controls genes such as immunity, inflammation, carcinogenesis, programmed death and infection. Therefore, the overactivation of NF-kB is expected to be involved in pathogenicity. Humans with underlying diseases such as liver disease and diabetes can develop lethal systemic diseases such as sepsis and necrotizing fasciitis. People with chronic liver disease are known to have activated NF-kB and NF-kB activation by the hot-PBS extract may exacerbate their conditions. The causative component in the hot-PBS extract is likely activated by the presence of fever in the patient and bacterial death caused by the infection, and this component may contribute to the development of severe necrotizing fasciitis Furthermore, the present study also suggested that NF-activating receptors other than NOD1 and NOD2 might exist. Future studies should attempt to isolate the activator from the hot-PBS extract. Once this goal has been achieved, the following items will require investigation: 1) structure determination, 2) contribution to pathogenicity and possible treatment targets, 3) search for receptors and construction of new innate immune mechanism, and 4) possibility of physiological activity and immunological control in animal models.
References
- Austin BC, Trinanes J, Escalona GN, et al. (2017). Non-cholera vibrios: The microbial barometer of climate change. Trends Microbiol. 25: 76-84.
- Jones MK, Oliver JD. (2009). Vibrio vulnificus: Disease and pathogenesis. Infect Immun. 77: 1723-1733.
- Horseman MA, Surani S. (2011). A comprehensive review of Vibrio vulnificus: An important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 15: e157-e166
- Mayer AM, Hall ML, Holland M, et al. (2014). Vibrio vulnificus MO6-24/O lipopolysaccharide stimulates superoxide anion, thromboxane B2, matrix metalloprotease-9, cytokine and chemokine release by rat brain microglia in vitro. Mar Drugs. 26: 1732-1756.
- Lee BC, Kim MS, Choi SH, et al. (2010). Involvement of capsular polysaccharide via a TLR2/NF-kappaB pathway in Vibrio vulnificus-induced IL-8 secretion of human intestinal epithelial cells. Int J Mol Med. 25: 581-591.
- Jeong HG, Satchell KJ. (2012). Additive function of Vibrio vulnificus MATRX(Vv) and VvhA cyolysins promotes rapid growth and epithelial tissue necrosis during intestinal infection. PLoS Pathog. 8: e1002581.
- Gavin HE, Beubier NT, Satchell KJ. (2017). The effector domain region of the Vibrio vulnificus MARTX toxin confers biphasic epithelial barrier disruption and is essential for systemic spread from the intestine. PLoS Pathog. 13: e1006119.
- Ogura Y, Inohara N, Benito A, et al. (2001). Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 276: 4812-4818.
- Westphal O, LÃÆÃâÃâüderitz O. (1954). Chemische Erforschung von Lipopolysacchariden gram-negativer Bakterien. Angew Chemie. 66: 407-417.
- Reddy GP, Hayat U, Abeygunawardana C. et al. (1992). Purification and determination of the structure of capsular polysaccharide of Vibrio vulnificus MO6-24. J Bacteriol. 174: 2620-2630.
- Gan L, Chen S, Jensen GJ. (2008). Molecular organization of gram-negative peptidoglycan. Proc Natl Acad Sci USA. 105: 18953-18957.
- Lawrence T. (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 1: a001651
- Luedde T, Schwabe RF. (2011) NF-kB in the liver-linking injury, fibrosis and hepatocellula carcinoma. Nat Rev Gastroenterol Hepatol. 8:108-118.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences