Hepatoprotective Effects of Deuterium Depleted Water(DDW)Adjuvant with Satureja rechingeri Essential Oils
Faezeh Fatemi, Abolfazl Dadkhah, Kambiz Akbarzadeh, Salome Dini, Shabnam Hatami, Azadeh Rasooli
1Nuclear Fuel Cycle Research School, Nuclear Science and Technology Research Institute, Tehran, Iran
2Department of Medicine, Faculty of Medicine, Qom Branch, Islamic Azad University, Qom, Iran
3Faculty of Medicine, Mashhad University of Medical Science, Mashhad, Iran
4Young and Elite Researchers Club, Karaj Branch, Islamic Azad University, Karaj, Iran
5Department of Physiology, Faculty of Science, Qom Branch, Islamic Azad University, Qom, Iran
6Department of Biochemistry, Faculty of Sciences, Payame-e-Noor University, Tehran, Iran
- Corresponding Author:
- E-mail: E-mail: ffatemi@aeoi.org.ir
Abstract
This study was carried out to investigate the hepatoprotective effect of deut erium depleted water (DDW) with and without Satureja rechingeri essential oil (E.O) mixture on paracetamol induced hepatotoxicity in rats. The animals were divided into 24 groups (n=5): The negative control group used tap water in 14 days following DMS O i.p injection at day 15th. The control group received tap water in 14 days following 500 mg/kg b.w. i.p injection of acetaminophen dissolved in DMSO. The treatment groups received only DDW (30 and 60 ppm) in 14 days concomitant with treatment groups receiving DDW plus i.p injection of S. rechingeri oil following acetaminophen injection at day 15th. Indeed, hepatoprotective activity was evaluated by the biochemical estimation of acetaminophen metabolism enzymes; cytochrome P450(CYP450), glutathione s-transferase (GST) together with the level of glutathione (GSH). The markers of liver injuries (ALT, AST, and ALP ) were also estimated in plasma. The results indicated that administration of DDW and DDW+E.O resulted in liver damage compensation as manifested by significant decrease in the activities of CYP450 and AST as well as significantly elevating the levels of GSH and GST. The present study reveals that the DDW could afford a significant protection against paracetamol? induced hepatocellular injuries.
Keywords
Satureja rechingeri; Deuterium depleted water (DDW); Metabolizing enzymes; Acetaminophen; Rat; Liver.
1. Introduction
Acetaminophen (Acetyl-para-aminophenol, APAP) is an analgesic and antipyretic drug well tolerated which widely used in therapy [1]. It can cause hepatic necrosis, nephrotoxicity, extra hepatic lesions and even death in humans and experimental animals when taken in overdoses [2]. Physiologically, APAP is primarily activated in the liver, by cytochrome P450 (CYP450) to the reactive metabolite N-acetyl-p- benzoquinone imine (NAPQI) [3,4]. At low doses, NAPQI is efficiently detoxified, principally by conjugation with GSH, a reaction catalyzed in part by the glutathione S-transferases (GSTs) [5]. But, conjugation at higher doses leads to the critical depletion of GSH by GST which is essential to maintain cellular redox metabolisms causing acute oxidative stress and cellular toxicity. There are numerous studies on the metabolic activation, toxicity and detoxification mechanism of APAP in the liver, kidney and other tissues [6-10].
Moreover, recent studies have proposed that one possible mechanism of APAP toxicity is the disturbance of prooxidant and antioxidant balance by generation of reactive oxygen species (ROS) and nitrogen species (RNS) [11-13]. There is increasing interest in the antioxidants of natural origin because they could suppress the oxidative damage of a tissue by stimulating the natural defense system. Consequently, application of natural antioxidants without any side effects for modulation of liver oxidative damages cannot be ruled out.
The natural water is a mixture of H2O and D2O, which the ratio between the hydrogen and deuterium atoms (R=H/D) is approximately 150 ppm. Light water called deuterium depleted water (DDW) has an isotopic ratio R smaller than 80ppm [14,15]. It has been noticed that a decreasing quantity of deuterium in drinking water has favorable effects on the organism such as anticancer properties [14]. On the other hand, Satureja rechingeri (S. rechingeri, Lamiaceae), an endemic species from Iran, is a well known medicinal plant for their long time healing properties and have been used as traditional folk remedies to treat various ailments such as cramps, muscle pains, nausea indigestion, diarrhea and infectious diseases [16-18]. Indeed, the genus has many biological activities, such as antibacterial, antifungal, and antioxidant properties [19,20].
There has still been a limited number of publications concerning biological activity of DDW especially hepatotoxicity activity. So, this research for the first time, aimed to evaluate the possible potential protective effect of DDW against parcetamol induced hepatotoxicity in rats. Furthermore, for evaluating more efficiency of DDW, its adjuvant with Satureja rechingeri essential oils were considered through measuring main hepatic metabolizing enzymes together with liver function markers.
2. Methods
Plant and DDW preparations
DDW was gifted from Atomic Energy Organization of Iran. In addition, fresh Iranian S. rechingeri was collected in summer from Khorramabad city, Iran. The plant material was authenticated by expert botanist.
Oil extraction and analysis
Oil extraction from the S. rechingeri aerial parts was carried out using a Clevenger-type apparatus. The extraction was carried out for 2 h and the essential oils were stored in dark glass bottles in a freezer until further use (1 month).
Gas chromatography (GC) analyses were performed using a Shimadzu-9A gas chromaph equipped with a flame ionization detector, and quantitative analysis was carried out on a Euro Chrom 2000 (Knauer) by the area normalization method, neglecting response factors. The analysis was carried out using a DB5 MS column (30 m × 0.25 mm, film thickness 0.25 μm). The operating conditions were as follows: injector and detector temperature, 250° and 280°C, respectively; carrier gas, Constant Flow. The oven temperature programme was 50–250°C at a rate of 7°C/minute. The GC/MS unit consisted of a Varian-3400 GC coupled to a Saturn II ion trap detector. The GC/MS column was the same as the GC under the same conditions that the above analysis was carried out. The identities of the oil components were established from their GC retention indices, relative to C7–C25 n-alkanes, by comparison of their MS spectra with those reported in the literature and by computer matching with the Wiley 5 mass spectra library, whenever possible, by co-injection with standards available in the laboratory [21,22].
Animal treatments
Male Wistar rats were used throughout this study. The animals were obtained from the Pasteur Institute of Iran and maintained in the animal house facilities. Adult animals were 3–4 months of age, weighing 150±20 g. They were maintained on a commercial pellet food and tap water ad libitum. The animals were divided into 24 groups (n=5). In negative control group (NC), the tap water was orally received in 14 days following i.p administration of APAP vehicle, i.e., 400 mL DMSO at day 15th. In control group (C), the acetaminophen (500 mg/kg b.w) dissolved in 400 mL DMSO was i.p injected at day 15th after receiving 14 day tap water. In the treatment groups, rats received orally only DDW (30 and 60 ppm) for 14 days along with rats drinked DDW (30 and 60 ppm) plus S. rechingeri essential oil (E.O) following acetaminophen administration at day 15th. The essential oils prepared from the plant at 20 mg/kg b.w were diluted in 400 mL DMSO and injected i.p in 14 days before acetaminophen treatment.
Preparation of tissue homogenate and plasma
The heparinated blood samples were collected at different time intervals (2, 4, 8, 16 and 24 h after APAP administration) by heart puncture from all the animals and centrifuged at 3000g for 10 min to obtain the plasma. Liver samples were immediately transferred to ice-cold containers and homogenized (20%, w/v) in the appropriate buffer using a homogenizer (E.L.M 2500). The homogenates were used to measure the biochemical parameters.
Biochemical assays
Cytochrome P450 protein assay: CYP450 protein level was performed by ELISA on liver preparations according to the procedure described in the kit from Bioassay Technology Laboratory, China.
GST protein assay: Liver cytosolic GST protein level was measured by ELISA as described in the instruction of the kit buying from Bioassay Technology Laboratory, China.
GST activity: Liver cytosolic GST activities were measured spectrophotometrically using CDNB as substrate as described by Habig et al. (1974). The specific activity was calculated based on the nmol/min//mg protein in samples which was measured by Bradford assay (Bradford et al., 1976). GSH estimation: GSH was estimated in tissue homogenates according to the procedure of Seldak and Lindsay (1986).
Liver function tests: to confirm the liver function and injury, serum alanine transminase (ALT), aspartate transminase (AST), alkaline phasphatase (ALP) and total bilirubin (BRN) were determined spectrophotometrically according to the procedure described in the kit purchased from the Pars Azmoon, Co, Iran.
Histopathological studies
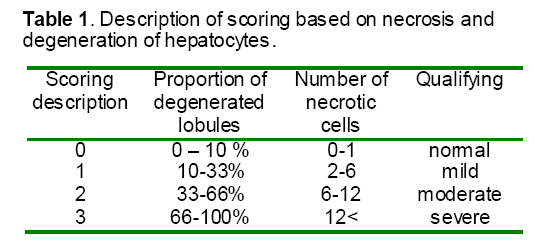
Small portions of livers were excised and placed into 10% freshly prepared formalin. After tissue processing, the samples sectioned at 6μm and stained. Severity of tissue injury index was based on vacuolar degeneration and necrosis of hepatocytes. The necrotic cells was determined by counting the numbers of these cells in10 randomly selected high power fields (400×) and scored as normal: 0 – 2, mild: 2 – 6, moderate: 6 – 12, severe: more than 12 by necrotic cell observations. The severity of vacuolar change was determined by estimating the proportion of lobules with vacuolated hepatocytes in 100× power field and scored as normal: 0 – 10 %, mild: 10 – 33 %, moderate: 33 – 66 %, severe: 66 - 100 %. Both scoring were described as 0: normal, 1: mild, 2: moderate, 3: severe that shown in Table 1.
Statistical analysis
Data are presented as means ± Standard Error of Mean (SEM) of five samples obtained from five animals in each group. The results were subjected to one-way ANOVA followed by Tukey’s HSD using SPSS (version 19.0) software. Significant levels were defined as P<0.05. (*) denote significantly different from the respective negative control group (P<0.05). (**) denote significantly different from the respective control group (P< 0.05).
3. Results
Essential oil analysis
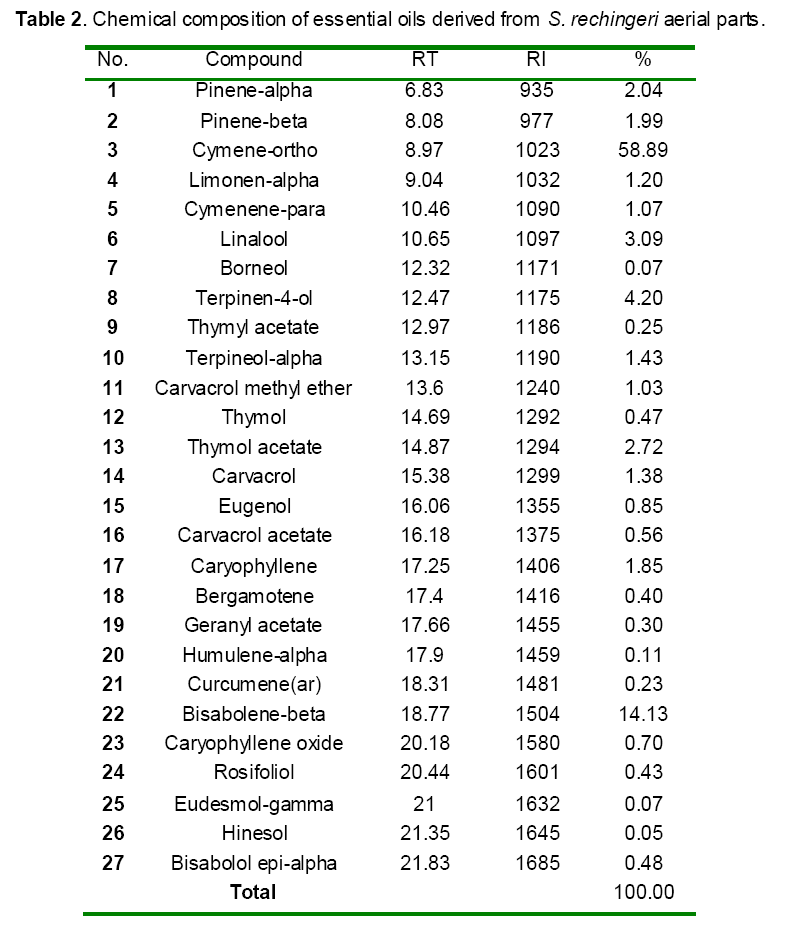
GC/MS analysis of S. rechingeri oils identified thirty-eight constituents with the major components as cymene-ortho (58.89%) followed by bisabolene-beta (14.13%) and terpinen-4-ol (4.20%) (Table 2). Remaining chemical compounds were in trace amounts.
Effect of pretreatment with the DDW and S. rechingeri essential oils against paracetamol induced hepatotoxicity on the hepatic GST and CYP450 and GSH levels
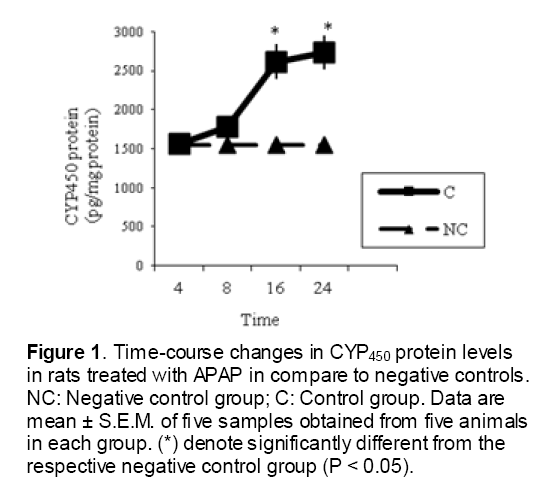
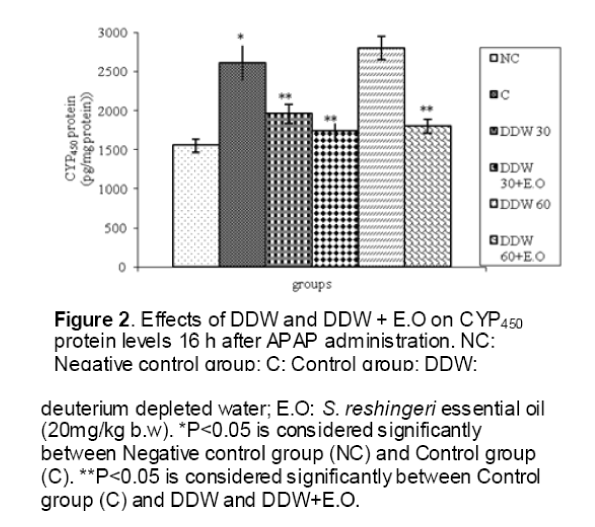
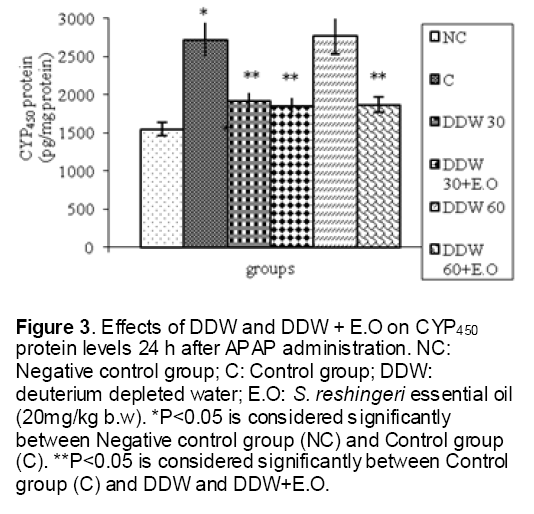
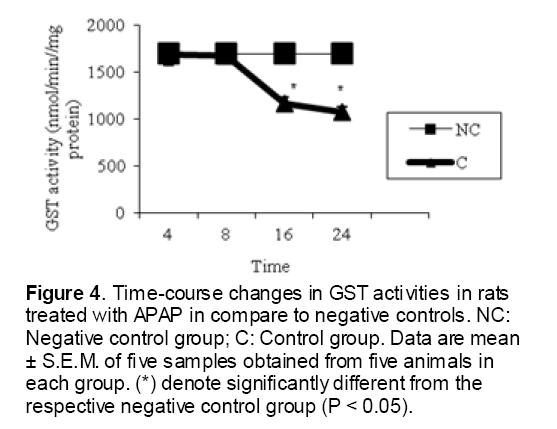
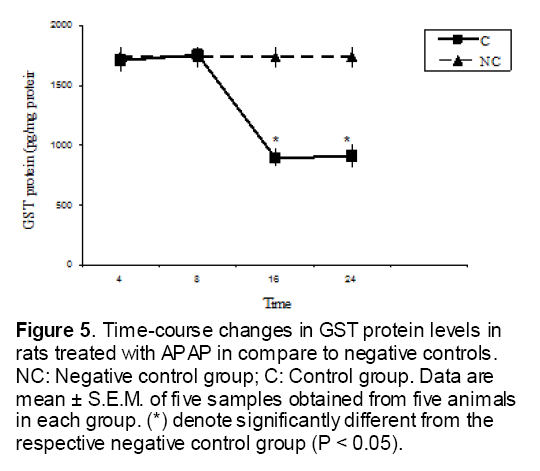
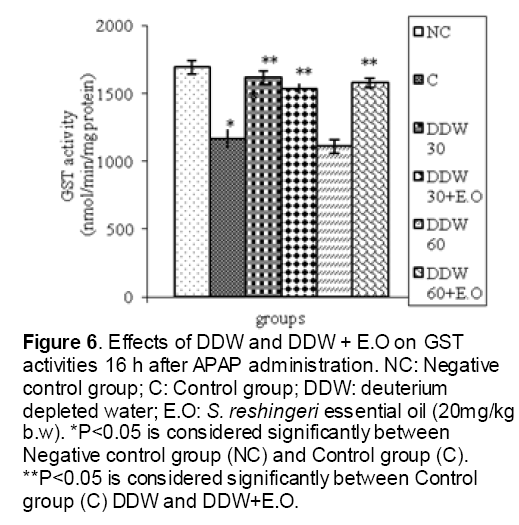
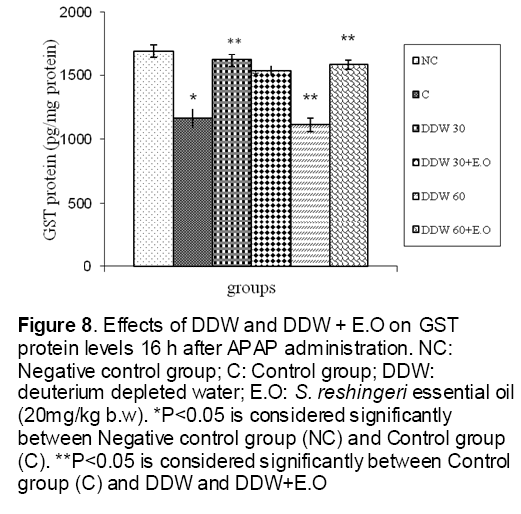
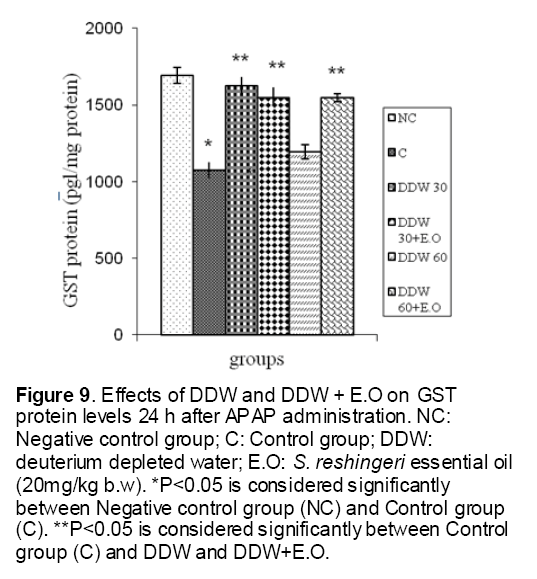
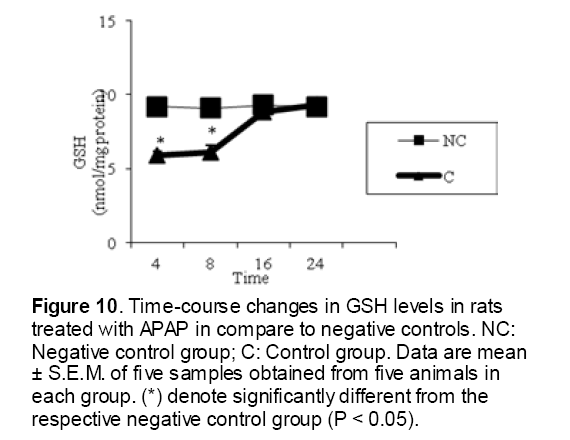
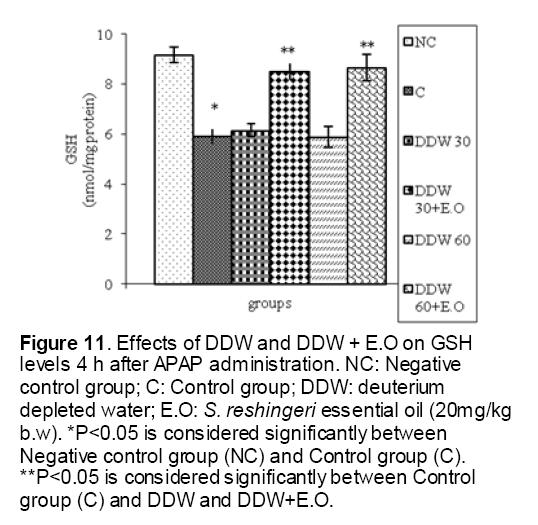
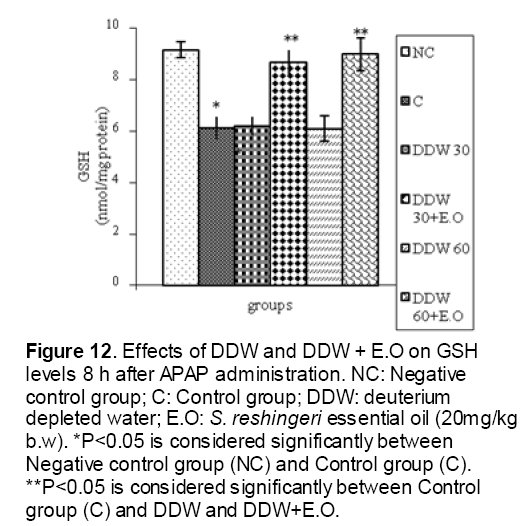
As shown in Figure 1, CYP450 protein level sig-nificantly (P < 0.05) increased in the liver of APAP-treated rats when compared to the untreated control at 16 and 24 h. Pretreatment of rats with DDW 30, DDW 30+E.O, DDW 60+E.O significantly (P < 0.05) reversed the serum levels of CYP450 to the normal level when compared to the positive groups at 16 and 24 h (Figure 2,3). The level of the detoxifying enzyme GST (both protein and activity) (Figure 4,5) was diminished in the rats treated with toxicity dose of APAP at 16 and 24 h (P<0.05). While, administration of DDW 30, DDW 30+E.O, DDW 60+E.O surprisingly elevated the reduction of serum GST activity and protein level induced by APAP (Figure 6,7 and Figure 8,9). Similarly, administration of APAP to rats led to a significant (P<0.05) decrease in liver GSH (4 & 8 h) as compared to the negative groups (Figure 10). Co-administration of rats with DDW 30+E.O, DDW 60+E.O could significant (P<0.05) restored the level of GSH towards normal value at (Figure 11,12).
Figure 1: Time-course changes in CYP450 protein levels in rats treated with APAP in compare to negative controls. NC: Negative control group; C: Control group. Data are mean ± S.E.M. of five samples obtained from five animals in each group. (*) denote significantly different from the respective negative control group (P < 0.05).
Figure 3: Effects of DDW and DDW + E.O on CYP450 protein levels 24 h after APAP administration. NC: Negative control group; C: Control group; DDW: deuterium depleted water; E.O: S. reshingeri essential oil (20mg/kg b.w). *P<0.05 is considered significantly between Negative control group (NC) and Control group (C). **P<0.05 is considered significantly between Control group (C) and DDW and DDW+E.O.
Figure 4: Time-course changes in GST activities in rats treated with APAP in compare to negative controls. NC: Negative control group; C: Control group. Data are mean ± S.E.M. of five samples obtained from five animals in each group. (*) denote significantly different from the respective negative control group (P < 0.05).
Figure 5: Time-course changes in GST protein levels in rats treated with APAP in compare to negative controls. NC: Negative control group; C: Control group. Data are mean ± S.E.M. of five samples obtained from five animals in each group. (*) denote significantly different from the respective negative control group (P < 0.05).
Figure 6: Effects of DDW and DDW + E.O on GST activities 16 h after APAP administration. NC: Negative control group; C: Control group; DDW: deuterium depleted water; E.O: S. reshingeri essential oil (20mg/kg b.w). *P<0.05 is considered significantly between Negative control group (NC) and Control group (C). **P<0.05 is considered significantly between Control group (C) DDW and DDW+E.O.
Figure 7: Effects of DDW and DDW + E.O on GST activities 24 h after APAP administration. NC: Negative control group; C: Control group; DDW: deuterium depleted water; E.O: S. reshingeri essential oil (20mg/kg b.w). *P<0.05 is considered significantly between Negative control group (NC) and Control group (C). **P<0.05 is considered significantly between Control group (C) and DDW and DDW+E.O.
Figure 8: Effects of DDW and DDW + E.O on GST protein levels 16 h after APAP administration. NC: Negative control group; C: Control group; DDW: deuterium depleted water; E.O: S. reshingeri essential oil (20mg/kg b.w). *P<0.05 is considered significantly between Negative control group (NC) and Control group (C). **P<0.05 is considered significantly between Control group (C) and DDW and DDW+E.O
Figure 9: Effects of DDW and DDW + E.O on GST protein levels 24 h after APAP administration. NC: Negative control group; C: Control group; DDW: deuterium depleted water; E.O: S. reshingeri essential oil (20mg/kg b.w). *P<0.05 is considered significantly between Negative control group (NC) and Control group (C). **P<0.05 is considered significantly between Control group (C) and DDW and DDW+E.O.
Figure 10: Time-course changes in GSH levels in rats treated with APAP in compare to negative controls. NC: Negative control group; C: Control group. Data are mean ± S.E.M. of five samples obtained from five animals in each group. (*) denote significantly different from the respective negative control group (P < 0.05).
Figure 11: Effects of DDW and DDW + E.O on GSH levels 4 h after APAP administration. NC: Negative control group; C: Control group; DDW: deuterium depleted water; E.O: S. reshingeri essential oil (20mg/kg b.w). *P<0.05 is considered significantly between Negative control group (NC) and Control group (C). **P<0.05 is considered significantly between Control group (C) and DDW and DDW+E.O.
Figure 12: Effects of DDW and DDW + E.O on GSH levels 8 h after APAP administration. NC: Negative control group; C: Control group; DDW: deuterium depleted water; E.O: S. reshingeri essential oil (20mg/kg b.w). *P<0.05 is considered significantly between Negative control group (NC) and Control group (C). **P<0.05 is considered significantly between Control group (C) and DDW and DDW+E.O.
Effect of pretreatment with the DDW and S. rechingeri essential oils against paracetamol induced hepatotoxicity on the liver enzymes
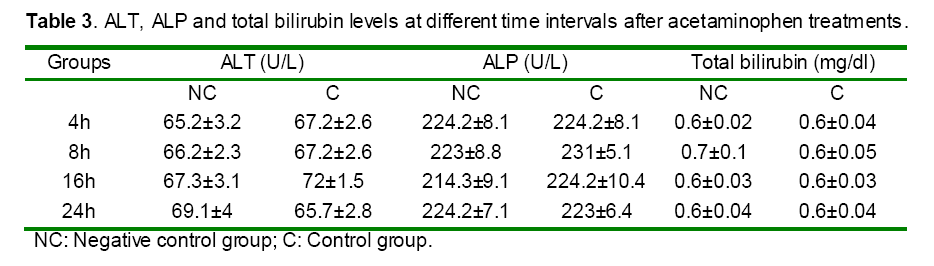
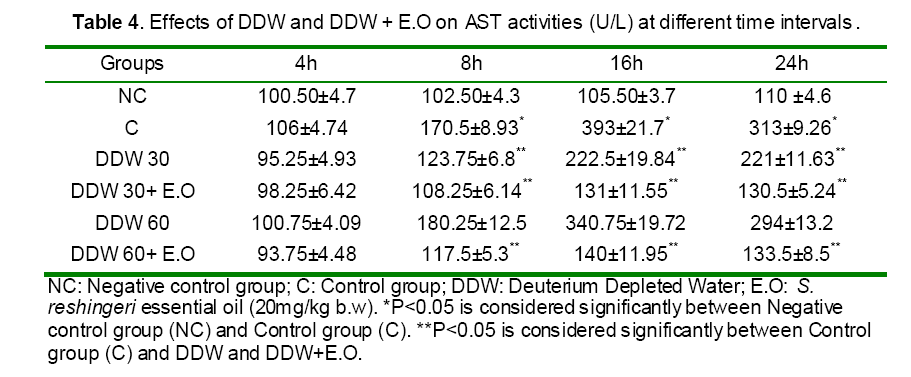
Serum activities of ALT, AST and ALP enzymes and serum bilirubin were given in Tables 3 and 4. Paracetamol significantly elevated the AST level in the intoxicated group at 8, 16 and 24h as compared to those of the control (non treated) group (Table 4). In contrast, rats pretreated with DDW 30, DDW 30+E.O, DDW 60+E.O significantly (P<0.05) protected the liver and decreased the AST level (8, 16 & 24 h) as compared to paracetamol intoxicated group (Table 4). However, the levels of ALP, ALT and total bilirubin was not significantly (P>0.05) changed in all time intervals as compared to negative control group (Table 3).
Histological findings
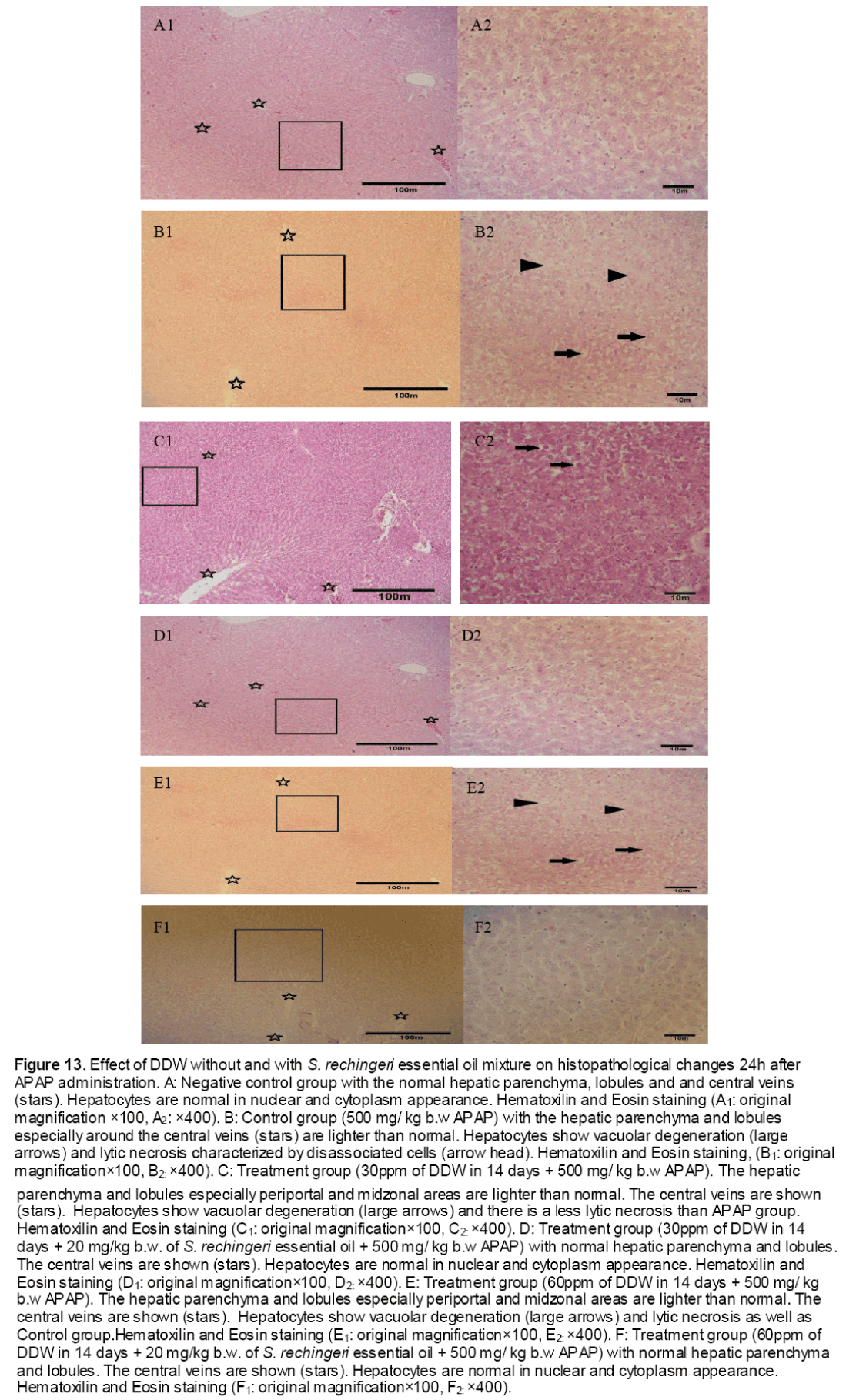
Histophatological studies performed on liver biopsies showed normal structure of the liver tissue in negative control group. There was no infiltration or sequestration of polymorphonuclear (PMN) or mononuclear inflammatory cells in this group (Fig. 13). The most severe pathologic lesions were seen in the groups 2 &5 received acetaminophen and acetaminophen + 60 ppm DDW. The livers were macroscopically larger and yellower than normal. Histopathological finding revealed severe multi focal necrosis, severe vacuolar degeneration of hepatocytes in the liver, scattered foci of infiltration of mononuclear inflammatory cells and to some extent mild mononuclear and neurophilic cholangitis in these groups. Also, treatment group received 30 ppm+ APAP revealed vacuolar degeneration in all livers but the rate of necrosis were only severe in 30% of rats. In addition, the groups received DDW plus essential oils were similar to some extent to negative control group. There were no evidences of bile retention in all treated groups.
Figure 13: Effect of DDW without and with S. rechingeri essential oil mixture on histopathological changes 24h after APAP administration. A: Negative control group with the normal hepatic parenchyma, lobules and and central veins (stars). Hepatocytes are normal in nuclear and cytoplasm appearance. Hematoxilin and Eosin staining (A1: original magnification ×100, A2: ×400). B: Control group (500 mg/ kg b.w APAP) with the hepatic parenchyma and lobules especially around the central veins (stars) are lighter than normal. Hepatocytes show vacuolar degeneration (large arrows) and lytic necrosis characterized by disassociated cells (arrow head). Hematoxilin and Eosin staining, (B1: original magnification×100, B2: ×400). C: Treatment group (30ppm of DDW in 14 days + 500 mg/ kg b.w APAP). The hepatic parenchyma and lobules especially periportal and midzonal areas are lighter than normal. The central veins are shown (stars). Hepatocytes show vacuolar degeneration (large arrows) and there is a less lytic necrosis than APAP group. Hematoxilin and Eosin staining (C1: original magnification×100, C2: ×400). D: Treatment group (30ppm of DDW in 14 days + 20 mg/kg b.w. of S. rechingeri essential oil + 500 mg/ kg b.w APAP) with normal hepatic parenchyma and lobules. The central veins are shown (stars). Hepatocytes are normal in nuclear and cytoplasm appearance. Hematoxilin and Eosin staining (D1: original magnification×100, D2: ×400). E: Treatment group (60ppm of DDW in 14 days + 500 mg/ kg b.w APAP). The hepatic parenchyma and lobules especially periportal and midzonal areas are lighter than normal. The central veins are shown (stars). Hepatocytes show vacuolar degeneration (large arrows) and lytic necrosis as well as Control group.Hematoxilin and Eosin staining (E1: original magnification×100, E2: ×400). F: Treatment group (60ppm of DDW in 14 days + 20 mg/kg b.w. of S. rechingeri essential oil + 500 mg/ kg b.w APAP) with normal hepatic parenchyma and lobules. The central veins are shown (stars). Hepatocytes are normal in nuclear and cytoplasm appearance. Hematoxilin and Eosin staining (F1: original magnification×100, F2: ×400).
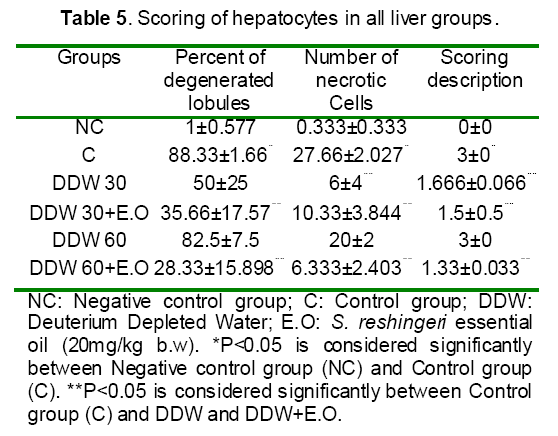
As shown in Table 5, most severe pathologic lesions as well as degenerated lobules and necrotic cells were shown in liver tissue of rats treated with acetaminophen. However, there were a significantly decrease in the number of degenerated lobules, necrotic cells and scoring description after administration of rats with DDW 30, DDW 30+E.O, DDW 60+E.O.
4. Discussion
Our current studies together with other studies have reported that the toxic effect of paracetamol was controlled in the animals treated with antioxidants of natural origin by the way of restoration of the levels of the antioxidant and detoxifying enzymes [23-27]. So, in the present study, paracetamol was employed as toxic agent and the protective effect of deuterium depleted water (30 and 60 ppm) without and with S. rechingeri essential oil mixture against the paracetamol induced hepatotoxicity was investigated.
Paracetamol is metabolized extensively in the liver. At therapeutic dose, about 80% of ingested paracetamol is eliminated primarily as sulfate and glucoronoide conjugate before oxidation and 5% is oxidized by hepatic cytochrome P450 to a highly reactive and toxic eletrophile, i.e. N-acetyl-p-benzoquineimine (NAPQI), and then conjugated with GSH by GST excreted in the kidney [28]. However, when high doses of acetaminophen are ingested, large amounts of reactive species known as N-acety-p-benzoquineimine (NAPQI) are produced which caused oxidative stress [28]. Furthermore, this metabolite also binds irreversibly to cellular macromolecules, an event often associated with hepatocellular damage, or it can be conjugated to reduce GSH by GST [10,29, and 30]. Our data confirmed these results indicating the abnormal levels of hepatic detoxification enzymes GST (Figure 4,5) and CYP450 (Figure 1) and also GSH (Figure 10) in rats induced by toxic dose of paracetamol (500 mg/kg b.w).On the other hand, when the hepatic cell membrane is injured, the liver enzymes GOT, GPT and ALT which are normally located in the cytosol, leak into circulation from hepatocytes leading to increased serum level of GOT, GPT and ALT [31]. As observed in the study, the level of serum enzymes AST was raised reflecting the hepatocellular damage due to paracetamol (Table 4) which was confirmed by histophatological examinations (Figure 13).
According to the disturbance of prooxidant and antioxidant balance by oxidative stress upon APAP toxicity, recent studies have proposed that many natural resources with antioxidant effects can participate in protection against paracetamol induced liver failure by scavenging reactive oxygen free radicals through different biochemical pathways [11,32]. The GSTs are a family of cytosolic enzymes involved in the detoxification of a range of xenobiotic compounds by conjugation their metabolite produced by CYP450 to glutathione which is essential in the maintenance of normal physiological processes [33]. Accordingly, in the present study, DDW 30, DDW 30+E.O and DDW 60+E.O DDW inhibit APAP induced liver damages as demonstrated by restoring the activities of CYP450 (Figure 2,3), GST (Figure 6,7 and Figure 8,9) and serum AST (Table 4). Also, the decreased hepatic GSH (Figure 11,12) in APAP treated rats is compensated by DDW 30+E.O and DDW 60+E.O DDW (P<0.05). These results were in accordance with our previous research found that the treatment of rats with Achillea wilhelmsii essential oils significantly modulate the biochemical parameters for instance, GST and CYP450 activities and GSH level to normal values [23]. Also, Kanchana and Sadiq mentioned that the protective effect of plumbago zeylanica on acetaminophen induced hepatotoxicity have been shown via lowering the respective serum AST, ALT, ALP and Albumin [34]. Similarly, rats pretreated orally with essential oil of Thymus Capitatus and Salvia Officinal intoxicated with paracetamol observed a significant protection against-induced increase in serum and hepatic LDH activities and inhibit reduce GSH levels and enhance increase SOD and GPx activities in blood and liver [35]. In connection, the protective effect of DDW (30 ppm) against chromoum intoxication in the liver organ by reaching the level of AST and ALT to those normal has been recorded [36].
As shown in Table 2, the essential isolated from Satureja rechingeri was characterized by cymene-ortho (58.89%) and bisabolene-beta (14.13%) as the principle compounds, possessed different antioxidant activities [37]. So, the possible mechanism responsible for the protection of the paracetamol induced liver damage may be the role of antioxidant compounds acted as a free radical scavenger by intercepting the radicals produced by microsomal enzymes in paracetamol metabolism. One study also demonstrated that the hepatoprotective effects of Kohautia grandiflora may be due to its antioxidant and free radical scavenging properties [38]. Additionally, the antioxidant property of DDW was probably effective in controlling of liver against acetaminophen toxicity. The difference between hydrogen and deuterium leads to differences in the physical and chemical behavior between the two stable isotopes. The concentration of deuterium being about 150 ppm in surface water and more than 10 mM in living organisms. But the concentration of deuterium in DDW is lower than 80 ppm which may possibly cause favorable effects on the organism [14]. Many studies established that the DDW may be used as adjuvant in the prevention or treatment of the different pathological states, especially in cancer for reducing cytostatic toxicity [39,40]. One study also reported that the DDW pre-treatment protects the liver from the chromium toxicity via scavenging effects) [36].
Conclusion
In conclusion, our results revealed that DDW and DDW+ E.O have a potent hepatoprotective effect upon acetaminophen toxicity in rats by reducing the formation and detoxification of the active intermediate APAP metabolite which was supported by estimating functional and histopathological characterization of liver sections.
References
- Ramsay R.R., Rashed M.S., Nelson S.D. (1989) In vitro effect of acetaminophen metabolites and analogs on the respiration of mouse liver mitochondria. Arch. Biochem. Biophys. 273(2): 449‐457.
- Ray S.D., Mumaw V.R., Raje R.R., Fariss M.W. (1996) Protection of acetaminophen-induced hepatocellular apoptosis and necrosis by cholesteryl hemisuccinole pretreatment. Journal of Pharmacology Experimental Therapeutics, 279(3): 1470-1483.
- Hinson J.A., Reid A.B., McCullough S.S., James L.P. (2004) Acetaminophen-induced hepatotoxicity: role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab Rev, 36(3-4): 805-822.
- Reid A.B., Kurten R.C., McCullough S.S., Brock R.W., Hinson J.A. (2005) Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther, 312: 509-516.
- Olaleye M.T., Kolawole A.O., and Ajele, J.O. (2007) Antioxidant Properties and Glutathione ST ransferases Inhibitory Activity of Alchornea cordifolia Leaf Extract in Acetaminophen-Induced Liver Injury. Iranian journal of pharmacology & therapeutics, 6(1): 63-66.
- Nelson, S.D. (2001) Molecular mechanism of the hepatotoxicity caused by acetaminophen. Semin liver Dis, 10(4): 267-278.
- Botta D., Shi S, White CC, Dabrowski MJ, Keener C.L., Srinouanprachanh S.L., FarinFM, Ware CB, Ladiges WC, Pierce RH, et al. (2006) Acetaminophen-induced liver injury is attenuated in male glutamate-cysteine ligase transgenic mice. J. Biol. Chem, 281(39): 28865-28875.
- Bajt M.L., Knight, T.R., Lemasters, J.J., Jaeschke H. (2004) Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci, 80(2): 343-349.
- Bessems J.G., Vermeulen N.P. (2001) Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches, Crit Rev Toxicol, 31: 55-138.
- Mitchell J. R., Jollow D. J., Potter W. Z., GilletteJ. R. & Brodie, B. B. (1973) Acetaminophen-induced hepatic necrosis; protective role of glutathione. J Phrm Exp Ther, 187: 211-217.
- James, L.P.; Mayeux, P.R., Hinson, J.A. (2003) Acetaminophen-induced hepatotoxicity. Drug Metab Dispos, 31(12): 1499-1506.
- Boobis A.R., Tee L.B., Hampden C.E., Davies D.S. (1986) Freshly isolated hepatocytes as a model for studying the toxicity of paracetamol. Food Chem Toxicol. 24: 731-736.
- Jaeschke H., Knight T.R., Bajt M.L. (2003) The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett, 144: 279-288.
- Olariu L., Petcu, M., Tulcan C., Chis-buiga I., Pup M., Florin M,. Brudiu, I. (2007) Deutrium depleted water –antioxidant or prooxidant, Lucrări stiinłifice medicină veterinară.TIMISOARA, 11: 265-269.
- Hăulică I., I. Păduraru ,B. Negu , I. Stefănescu ,I. TiŃescu – (1988) Possible antioxidant properties of the deuterium depleted water, Progress of Cryogenics and Isotopes Separation, Calimanesti.
- Bezic N., Ivica Š., Valerija B., Višnja B., Jasna P. (2009) Essential oil composition and internal transcribed spacer (ITS) sequence variability of four south-Croatian Satureja species (Lamiaceae). Molecules,14: 925-938.
- Jamzad, Z. (1996) Satureja rechingeri (Labiatae), a new species from Iran. –Annalen Des Naturhistorischen.
- Senatore F., Urrunaga Soria E., Urrunaga Soria R., Della Porta G, De Feo V. (1998) Essential oils from two Peruvian Satureja species.Flavour and Fragrance, 13(1): 1-4.
- Azaza D., Demircib F., Satila F., Kürkcüoglub M., and Baser K. H. C (2001) Antimicrobial activity of some Satureja essential oils. Z. Naturforsch. 57: 817-821.
- Güllüce M., Sökmen M., Daferera D., Ag˘ar G., Ozkan H., Kartal N., Polissiou M., Sökmen A., Sahin F. (2003) In vitro antibacterial, antifungal, an antioxidant activities of the essential oil and methanol extracts of herbal parts and callus cultures of Satureja hortensis L. J. Agric. Food Chem, 51(14): 3958-3965.
- Shibamoto T. (1987) Retention indices in essential oil analysis. In: Capillary Gas Chromatography in Essential Oil Analysis, Edits., P. Sandra and C. Bicchi, 259–274, Dr.Alfred Huethig Verlag, Heidelberg.
- Davies N.W. (1990) Gas chromatographic retention index of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20 M phases. J. Chromatogr, 503: 1-24.
- Dadkhah A., Fatemi F., Ababzadeh, S., Roshanaei K., Alipour M., Sadegh Tabrizi B. (2014) Potential preventive role of Iranian Achillea wilhelmsii C. Koch essential oils in acetaminopheninduced hepatotoxicity. Botanical Studies, 55:37.
- Dadkhah A., Fatemi F., Eslami Farsani M., Roshanaei K., Alipour M., Aligolzadeh H. (2014) Hepatoprotective Effects of Iranian Hypericum scabrum Essential Oils Against Oxidative Stress Induced by Acetaminophen in Rats. Braz. Arch. Biol. Technol. 57(3): 340-348.
- Dadkhah, A, Fatemi, F, Alipour, M, Fatourehchi, S, Sadat Parchini, F. (2015) Regulatory effect of Iranian Hypericum Scabrum essential oils on hepatic metabolizing enzymes in rats treated by acetaminophen, Journal of Essential Oil Bearing Plants, 18(2): 335-348.
- Dadkhah A., Fatemi F., Alipour M., Ghaderi Z., Zolfaghari F., Razdan F. (2015) Protective effects of Iranian Achillea wilhelmsii essential oil on acetaminophen induced oxidative stress in rat liver, Pharmaceutical Biology, 53(2): 220-227.
- Nithianantham K., Shyamala M., Chen Y., Latha L.Y., Jothy S.L., Sasidharan S. (2011) Hepatoprotective potential of Clitoria ternatea leaf extract against paracetamol induced damage in mice. Molecules. 6(12): 10134-10145.
- Jose J.K., Kuttan R. (2000) Hepatoprotective activity of Emblica officinalis and Chyavanaprash. Journal of Ethnopharmacology, 72: 135-140.
- Mitchell J.R., Jollow D.J., Potter W.Z., Davis D.C., Gillette J.R. (1973) Acetaminophen-induced hepatic necrosis; role of drug metabolism. J Phrm Exp Ther, 187(1): 187-194.
- Mudge G.H., Gembory M.W., Duggin, G.G. (1978) Covalent binding of metabolites of acetaminophen to kidney protein and depletion of renal glutathione. J Pharmcol Exp Ther, 206(1): 218-226.
- Arote S., Gupta S.K., Lodha P., Yadav P., Man Lodha L. (2014) Hepatoprotective activity of herbal formulation against paracetamol-induced hepatotoxicity in rats. indo american journal of pharmaceutical research, 4(1): 451-456.
- Kumari A., Kakkar P. (2012) Lupeol prevents acetaminophen-induced in vivo hepatotoxicity by altering the Bax/Bcl-2 and oxidative stress-mediated mitochondrial signaling cascade. Life Sci, 90(15-16): 561-570.
- Hayes JD, Pulford DJ., (1995) The glutathione-S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 30: 445-600.
- Kanchana N., Mohamed sadiq A. (2011) Hepatoprotective effect of plumbago zeylanica on paracetamol induced liver toxicity in rats. Int J Pharm Pharm Sci, 3(1): 151-154.
- El-Banna H., Soliman M., Al-wabel N. (2013) Hepatoprotective Effects of Thymus and Salvia Essential oils on Paracetamol-Induced Toxicity in Rats, J. Phys. Pharm. Adv., 3(2): 41-47.
- Doina, P.M, Victor Olariu, V, Scurtu, M, Tulcan, C, Ileana Brudiu, I, Muntean, D, Petcu, F, Pădeanu, I, Ostan, M. (2012) The effect of deuterum depleted water on some hepatic enzymes’ activity in rats intoxicated with chromium (vi), fascicula: ecotoxicologie, zootehnie si tehnologii deindustrie alimentară, 521-526.
- Sefidkon F., Abbasi K., Jamzad Z., Ahmadi S. (2007) The effect of distillation methods and stage of plant growth on the essential oil content and composition of Satureja rechingeri Jamzad. Food chemistry, 100 (3): 1054-1058.
- Garba S. H., Sambo N., Bala U. (2009) The effect of the aqueous extract of kohautia grandiflora on paracetamol induced liver damage in albino rats. Nigerian Journal of Physiological Sciences, 24(1): 17 -23.
- Goodall K. (2003) The Role of Deuterium in DNA degradation, Anti-Aging Medical News, Resource.
- Mirică R.E. (2010) Deuterium - depleted water in cancer therapy, Environmental Engineering and Management Journal, 9(11): 1543-1545.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences