Golden Natural Plant Compounds Activate Apoptosis via Both Mitochondrial and Death Receptor Pathways: A Review
Mohammad Firoozinia, Soheil Zorofchian Moghadamtousi, Aidin Sadeghilar, Hamed Karimian, Mohamed Ibrahim Bin Noordin
1Biomolecular Research Group,Biochemistry Program,Institute of Biological Sciences,Faculty of Science,University of Malaya,50603 Kuala Lumpur,Malaysia
2Department of Orthopaedics,Royal Children's Hospital,University of Queensland,Brisbane,Australia
3Department of Pharmacy,Faculty of Medicine,University of Malaya,50603 Kuala Lumpur
Received: October 26,2015; Accepted: October 31,2015; Published: November 03,2015
Abstract
Apoptosis (programmed cell death) is an intricate process that involves multiple signaling pathways, and failure to activate apoptosis is one of the main obstacles in cancer treatment. The apoptosis resistance of cancer cells is an inherent part of the carcinogenic process, which is also associated with chemotherapy resistance. Therefore, new drugs are required to improve cytotoxicity to cancer cells without affecting normal cells. The current knowledge of apoptosis is the theoretical source for novel and effective treatments with the selective induction of apoptosis in cancer cells and augmentation of the cytotoxicity of established chemotherapeutic agents. Based on these goals of treatment for cancer, recent attention has focused on the phytochemicals as anticancer agents. For that reason, designing new anti-tumor agents must include recognizing and developing those agents that have the ability to target various genes that regulates apoptosis. There are several natural plant compounds that demonstrate anti-tumor activity, according to various mechanisms. In view of the immense medicinal importance of natural products, this review strives to compile a brief statement on the mechanisms of apoptosis and effective natural compounds that induce apoptosis through both mitochondrial and death receptor pathways.
Keywords
Natural compounds; Apoptosis; Cancer; Mitochondria; Death receptor.
Introduction
Apoptosis
As an extremely structured process of programmed cell death,apoptosis has developed much attention in oncology and cancer therapy due to the role of chemotherapeutic agents in encouraging apoptosis in cancer cells [1]. In response to cancer treatment,apoptosis is one of the main mechanisms of planned cell death [2]. Apoptosis is an evolutionary procedure that naturally occurs in all cells that are no longer useful [3]. Apoptosis has an important role in physiology,homeostasis and development [4-7]. The deregulation of apoptosis through gain of antiapoptotic signals or the loss of pro-apoptotic signals may indicate a variety of pathological circumstances,resulting in treatment failure or cancer promotion,progression and initiation [8,9]. Apoptosis is a desirable process throughout the duration of cancer treatments because it does not frequently activate an immune or inflammatory response. In this way,the induction of apoptosis using chemical agents and the synthesis of apoptotic pathways is effective in cancer therapies [10-15]. Activating various caspase cascades can mediate the apoptosis process [16,17]. There are two main signaling systems in mammals,that result in the caspase activation; the mitochondrial pathway (intrinsic) [18,19],and the death receptor pathway (extrinsic) [20-23]. The extrinsic pathway involves death receptors,such as the tumor necrosis factor receptor or Fas,and the procaspase- 8 undergoes fission by the active system following the activation of downstream caspases-3,-6 and -7 [24]. In the intrinsic pathway,indispensable roles are performed by mitochondria over the mitochondrial permeability transition (PT). Mitochondrial PT induction indicates the destruction of the internal transmembrane prospective (Dwm),an accepted mechanism that activates cytochrome c translocation [25]. There are various anti-apoptotic molecules available that have the ability to regulate or trigger apoptosis. Thus,it has become a significant approach for cancer chemotherapy to utilize unindustrialized natural antitumor compounds that have the capability to target these molecules. Currently,many plant compounds have been acknowledged as anticancer agents [26],and many can activate apoptosis by targeting several cellular proteins,encouraging apoptosis via both the extracellular and intracellular pathways [14-27] (Figure 1).
Intrinsic apoptosis
The intrinsic apoptosis signaling pathway includes intracellular non-receptor–mediated signals,which are active in the mitochondria [28]. The intrinsic pathway stimuli consist of cells that have been injured by toxins or virus-related infections,radiation or free radicals. Additionally,cellular DNA damage can encourage the activation of the intrinsic pathway. Variations in the internal mitochondrial membrane are induced through these stimuli,and the outcome is the loss of transmembrane potential as well as the stimulation of pro-apoptotic proteins in the cytosol [28,29]. Caspases are activated by pro-apoptotic proteins,which mediate the destruction of the cell over countless pathways. A hallmark of apoptosis induced by DNA fragmentation is the translocation of those proteins into the cellular nucleus [28]. The tumor suppressor protein p53 and Bcl-2 protein family members are the main reasons for the regulation of pro-apoptotic actions. Protein members of the Bcl-2 group may play both pro- or anti-apoptotic roles [28]. The anti-apoptotic proteins contain BAG,Bcl-x,Bcl-XS,Bcl-XL,Bcl-w and Bcl-2,and many of these proteins are presently being explored as latent targets for anti-tumor therapy [28]. In addition,Bim,Bik,Bak,Bid,Bad,Bax,Bcl-10,and black are pro-apoptotic proteins. Furthermore,p53 protein and the cellular pathways that regulate its function are also presently being investigated as targets for prospective anticancer therapies [30] (Figure 2 ).
Extrinsic apoptosis
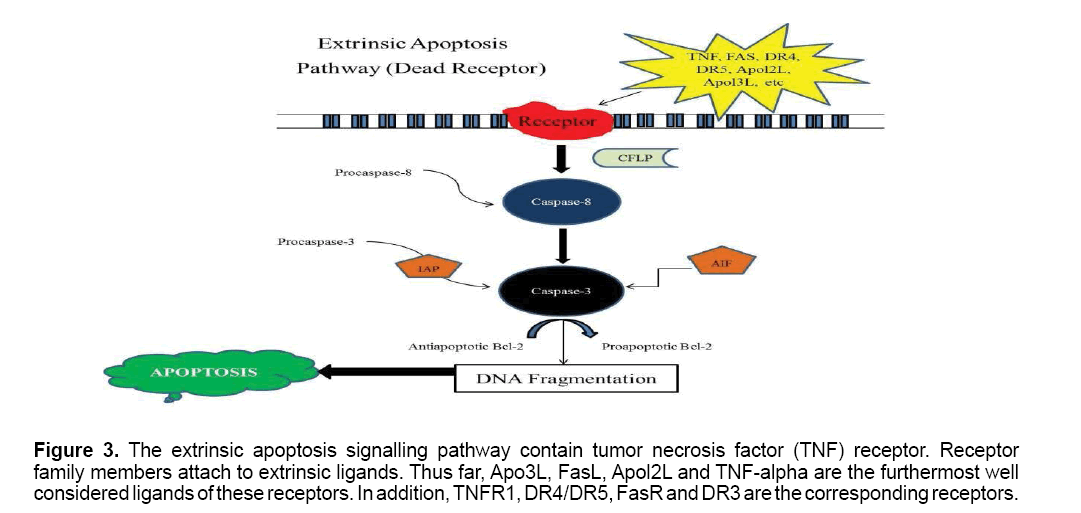
The extrinsic apoptosis signaling pathway is associated with tumor necrosis factor (TNF) receptor gene superfamily,such as transmembrane death receptors. Receptor family members attach to extrinsic ligands,which ultimately result in damage to the cell due to the transduction of intracellular signals [31,32]. Thus far,Apo3L,FasL,Apol2L and TNF-alpha are the most well investigated ligands of these receptors. In addition,TNFR1,DR4/DR5,FasR and DR3 are the corresponding receptors [31,33]. Currently,the molecules that may activate these receptors or encourage the activities of these pro-apoptotic proteins are being investigated for their possible therapeutic potential for cancer treatment. A number of caspases,which are mostly proteases with particular cellular targets,play a role in extrinsic pathway signal transduction. Once activated,caspases- modify a number of cellular functions that result in cell death [33] (Figure 3 ).
Figure 3: The extrinsic apoptosis signalling pathway contain tumor necrosis factor (TNF) receptor. Receptor family members attach to extrinsic ligands. Thus far, Apo3L, FasL, Apol2L and TNF-alpha are the furthermost well considered ligands of these receptors. In addition, TNFR1, DR4/DR5, FasR and DR3 are the corresponding receptors.
Natural compounds
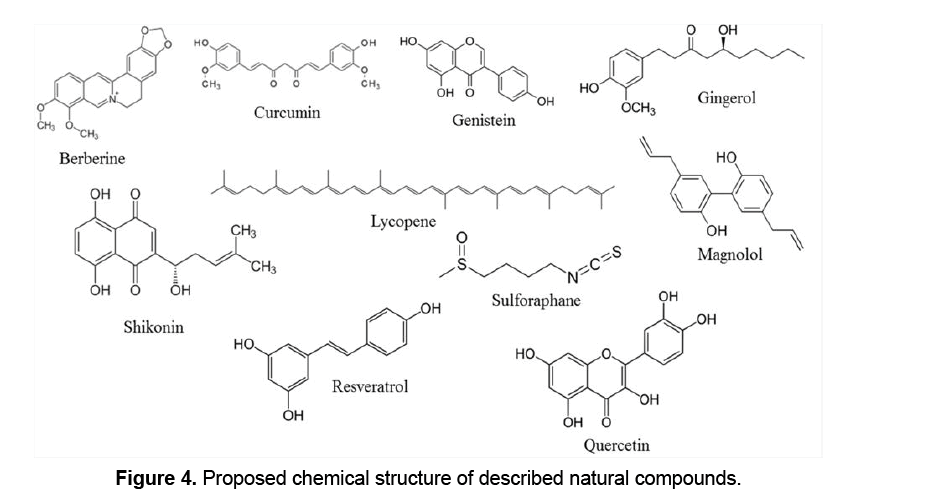
The latest information on malignant tumor repressive compounds of plant origin has generated an impressive range of unique constructions. Epidemiological studies have suggested that the intake of a diet with adequate vegetables and fruits,which are the main sources of micronutrients and phytochemicals,may decrease the possibility of cancer development [34]. It was demonstrated that specified products of plants encourage apoptosis in neoplastic but not in normal cells [35-37]. Apoptosis has been increasingly suggested as an imperative approach of action for various anti-tumor mediators [38,39]. Therefore,the study of plant-based apoptotic inducers is considered essential,whether as isolated components or as crude extracts. A unifying concept for the mechanism may signify chemoprevention the definitive capability of plant compounds to encourage apoptosis. Thus,study of the action of these compounds may provide useful evidence of the possible application of these compounds in the prevention of cancer or even in cancer therapy (Figure 4 ).
Berberine
Berberine is derived from proto-berberine as a quaternary ammonium salt of isoquinoline alkaloids. It is used for treating intestinal parasite and bacterial diarrhea infections as a traditional medicine. It was recently shown to suppress the growth of a number of tumor cell lines. Berberine reduced colony development of colon tumors in agar,and encouraged LDH release and cell death in a concentration- and time- dependent method in IMCE cells [40]. In a ROS production-dependent method,berberine motivated nuclear translocation,which is an intermediate of cell death (caspase-independent) and promotes apoptosis-inducing factor (AIF) release from mitochondria. Enhancement of berberinemotivated ROS suppression or the production of AIF in IMCE cells releases LDH and blocks berberineinduced apoptosis [40]. Moreover,as two targets of ROS production in cells,PARP initiation and the diffusion of the lysosome cathepsin B were encouraged through berberine. Obstruction of any of these pathways reduced cell death and berberineinduced AIF activation in IMCE cells. Therefore,berberine- motivated PARP activation-dependent AIF activation and ROS construction,promoting the release of cathepsin B,which promoted caspaseindependent colon tumor cell death [40]. In addition,Berberine induces apoptosis through procaspase-9 in liver cancer cells,and through procaspase-7 and procaspase-3,which are its effector caspases.
The reverse transcription-PCR (polymerase chain reaction) results showed that berberine amplified the expression of Bax,resulting in the activation of the caspase cascade. The existing definitions proved that berberine encourages apoptosis in Huh7 cells using the mitochondrial pathway [41]. Previous studies established berberine’s ability to induce cell cycle arrest,inhibit inflammation and promote apoptosis in colon carcinoma cells. Supported by the induction of p21 expression at the G2/M phase,berberine arrested the SW480 cell cycle. Sequences of biochemical procedures,containing caspases,the release of cytochrome-c to the cytosol,the cleavage of poly (ADP-ribose) polymerase (PARP),the loss of mitochondrial membrane potential and Bcl-2 family protein induction suggests that berberine has the ability to induce apoptosis. Additionally,as shown by tumor necrosis factor- related apoptosis-inducing ligand (TRAIL) expression,and vascular endothelial growth factor (VEGF),caspase-8 mediated angiogenesis was inhibited by berberine [42]. A significant decrease of apoptosis caused by HIF-1α and p53 was found in the cerebral tissue of MCAO rats treated with berberine [43].
Genistein
Genistein has been presented to have anticancer activity as a natural isoflavonoid phytoestrogen,by promoting cell cycle arrest (G2M phase),and it may influence apoptosis in a number of cancer cell lines. It has been suggested that treatment using genistein promoted both the activation of caspases via Bid truncation (tBid) and the down-regulation of cellular caspase-8 (FLICE)-like inhibitory protein [44]. The significant character of caspases in apoptosis,promoted by genistein has shown the apoptotic effects of co-treatment with genistein which were meaningfully inhibited by particular caspase inhibitors.
Overall,the published results specify that genistein can potentiate apoptosis via the up-regulation proapoptotic tBid proteins as well as cFLIP∧L downregulation [44]. Genistein dramatically enlarged its exploitive consequence on both angiogenesis and tumor growth in nude mice,and potentiated the apoptosis-inducing consequences of ATO and the in vitro inhibition of the proliferation of HCC cell lines. The mechanism is partly based on the exploitive consequences of genistein on both the ATO-induced,activation of Akt,and on the activity of the NF-κB,in addition to the final association through the of NF-κB regulated suppression of gene products,such as BclxL,VEGF,Bcl-2,COX-2,cyclin D1,and c-myc [45]. Genistein suppresses apoptosis in moto-neurons via pro-inflammatory cytokines such as IFN-γ in microglia. In vitro,exposure of ventral spinal cord 4.1 motoneurons to microglial cytokine supernatant produced substantial apoptosis,which impacted the potential of the mitochondrial membrane. In addition,proliferation has been noted in species of reactive oxygen,calpain,intracellular Ca2+,cytochrome c,bax:bcl-2 ratio,and caspases. Microglial cytokine insult resulted in inverted moto-neuron apoptosis using genistein [46]. Records specified that genistein encourages cellular death in LNCaP cells through the intrinsic apoptotic pathway via the activation of caspase-3 and caspase-9 [47]. Furthermore,genistein has been shown to inhibit the propagation of MCF-7 HER2 in addition to MCF -7 vec cells,and this growth inhibition was increased with a rise of subG0/ G1 apoptotic elements. Genistein up-regulates p53,which induces the extrinsic apoptosis pathway [48].
Curcumin
Curcumin is a well-known food coloring and cooking agent from the turmeric rhizome (Curcuma longa L.),and it has therapeutic effects against various pathologies such as atherosclerosis,heart failure and cancer [49]. Curcumin possesses potent antitumor,antioxidant and anti-inflammatory activities,and it has the ability to induce apoptosis in HL-60 promyelocytic leukemia cells. Apoptosis induced by curcumin could be noticeably decreased by the proteinase inhibitor tosyl lysine chloro-methyl ketone (TLCK) or an endonuclease inhibitor (ZnSO4),whereas the partial effect was shown by TPA (12-O-tetradecanoylphorbol-13-acetate). The antioxidants,alpha-tocopherol,l-ascorbic acid,superoxide dismutase and catalase,all efficiently prohibited apoptosis induced by curcumin. The results of a prior study suggested that cell death induced by curcumin was facilitated by species of reactive oxygen [50]. Curcumin induced apoptosis in NCI-H460 cells together with morphological changes in a dose-dependent manner. These signals culminate in caspase-3 activation and loss of mitochondrial membrane potential (ΔΨm). Apoptosis induced by curcumin was also encouraged over the ER stress proteins,GRP78 (glucose-regulated protein 78),FAS/caspase-8 (extrinsic pathway),and GADD153 (DNA damage-inducible gene 153),and growth arrest in NCI-H460 cells was activated [51]. It has been reported that curcumin has an antitumor effect by suppressing the growth of tumor cells and inducing apoptosis. Previous studies determined that curcumin induces apoptosis via a miRNA pathway in A549 cells [52]. Curcumin could activate p38-MAPK (p38-mitogen-activated protein kinase) and c-jun NH2 terminal kinases (JNKs). Curcumin up-regulated ROS generation and triggered the activation of JNKs,which encouraged H9c2 apoptotic cell death [49]. In addition,studies reveal that the JNK/ERK/AP1 pathway activation by curcumin can encourage THP- 1 cell apoptosis [53].
Gingerol
Gingerol (also known as 6-gingerol),is the active component of fresh ginger. This compound is a relative of piperine and capsaicin [54]. It has been reported that gingerol (6-Gingerol),which is the strongest ingredient found in the rhizome of ginger,has strong anti-inflammatory properties,which is closely related to its cancer chemo preventive property. The scientific literature confirms that ginger-derived compounds have inhibitory effects in several types of cancer cells [55]. Gingerol inhibited Bcl-2 expression,caused DNA fragmentation and encouraged apoptosis of HL-60 promyelocytic leukemia cells [56]. The mechanism of 6-gingerol-induced apoptosis might account for the inhibition of Bcl-2 expression in HL-60 cells [56]. It has been proven that mitochondria and lysosomes in HepG2 cells may possibly be the main targets of gingerol (6-gingerol). The 6-gingerol-induced apoptosis oxidative stress has been determined and the intracellular generation of reactive oxygen species (ROS) and reduced glutathione (GSH) were observed,which suggests that the positive mediator of 6-gingerol that induces apoptosis in HepG2 cells may be cathepsin D [57]. Additionally,6-gingerol may possibly encourage apoptosis in human prostate cancer cells (LNCaP) [58]. The mitochondrion is the key organelle that provides energy to cells for their growth and survival. Mitochondria have a central role in apoptosis of cancer cells and may also regulate autophagy [59]. Further,the expression of caspase-3 was distinguished in prostate cancer cells,which is preserved by 6-gingerol [60]. The caspases play a fundamental role in the apoptotic process as a family of proteins. These exist inside the cells as inactive precursors,which belong to a set of enzymes recognized as cysteine proteases [61]. Caspase trigger activation in cells undergoing apoptosis has been shown in both the extrinsic and intrinsic pathways [62]. The appearance cleaved PARP by 6-gingerol treatment was associated with the appearance of caspase-3 [60]. Additionally,6-gingerol has produced possibility reduction in gastric carcinoma cells [63]. Furthermore,caspase- 3-dependent cell death and cell cycle arrest was encouraged by 6-gingerol in colorectal carcinoma cells [64]. In conclusion,identified data recommend that 6-gingerol might be industrialised as one of the operational chemotherapeutic or chemopreventive mediators in several cancers [64]. The caspase group of cysteine proteases is involved in the death of cells in response to several apoptotic mechanisms via death-receptor (extrinsic) and mitochondria dependent pathways (intrinsic) [65]. A variety of substrates including activated DNase and mechanisms of cellular DNA repair are cleaved by caspases [66]. As detected by TUNEL assay in the prostate of mice,the 6-gingerol activated caspase -9 and -3,resulting in DNA fragmentation [66].
Lycopene
Tomatoes,as well as other vegetables and red fruits (except cherries,red bell peppers and strawberries),are the sources of lycopene,a carotenoid pigment (bright red carotene). Lycopene is concentrated in the prostate gland,where it is believed to act as an antioxidant,and it is also responsible for other protective roles in battling prostate cancer [67]. Lycopene has the ability to decrease colon cancer,lung carcinomas and adenomas,breast (mammary) tumors,and prostate cancer,and to inhibit HL-60 leukemic cell growth as well as endometrial cancer. Analysis of tumor-suppressor protein alterations suggested that lycopene promotes cell cycle arrest. Treatment with lycopene transformed proteins complicated the apoptosis signaling pathway,together with the appearance of Caspase-3,cleaved PARP and Bax/Bcl-xL in LNCaP cells [68]. Cardiomyocyte pretreatment with lycopene inhibited the initiation of the mitochondrial permeability transition pore (mPTP) by decreasing the intracellular reactive oxygen species (ROS) stages and stopping the proliferation of malondialdehyde (MDA) levels. The loss of mitochondrial membrane potential has been observed in lycopene-treated cultures,as well as a reduction in the levels of cellular ATP,caspase-3 activation and a decrease in the volume of cytochrome c translocated to the cytoplasm. It has been suggested that lycopene has pharmacological potential in protecting H/R-induced apoptosis [69]. Early studies suggested that the antioxidant effect of lycopene may reduce the severity of chronic diseases such as cancer. In vitro,lycopene is the most proficient carotenoid singlet oxygen neutralizing component in nature [70]. Lycopene generates apoptosis and motivates the intrinsic pathway concerning the mitochondrial release of cytochrome C,in addition to Annexin V exposure. Lycopene anti-proliferation activity is frequently recognized together with the capability of the molecule at the G0/G1 phase to block the cell cycle [71]. Lycopene promotes apoptosis by reducing Bcl-2 and BclXL,increasing the levels of the pro-apoptotic proteins Bax,Bad,Bim,and Fas ligand,and activating caspases 8,9,and 3 (right panel). It can also block growth factor-mediated antiapoptotic signals by directly inhibiting the binding of growth factors to their receptors or by inhibiting the downstreamPI3K-AKTpathway. Lycopene can promote apoptosis and AKT-induced cell-cycle arrest through the inactivation and phosphorylation of GSK3β,p21,p27,caspase 9,and Bad,as well as the inactivation of p53 via Mdm2 [72].
Magnolol
Magnolol is a component of traditional Asian herbal teas,which is a hydroxylated biphenyl mediator sequestered from Magnolia plants. This component has been stated to have anti-cancer,antiinflammatory,and anti-microbial activity. It was shown in non-small lung cancer cells (NSCLC) that magnolol has the capability to increase DNA fragmentation,inhibit cellular proliferation,and reduce the potential of the mitochondrial membrane. Magnolol generated the release of the pro-apoptotic proteins Bax,Bid,and cytochrome c from mitochondria; however,it did not do the same for caspase-3,-8,and -9,signifying the promotion of apoptosis by magnolol over a caspase-independent pathway in NSCLC cell lines [73]. Endonuclease G and cleaved poly (ADP-ribose) polymerase as well as the nuclear translocation of apoptosis-inducing factor,which mediates the caspase-independent pathway,played significant roles in mediating cell death. Additionally,magnolol inhibited the activity of ERK1/2 and PI3K/ AKT,and up-regulated JNK activity and p38 in A549 cells [73]. Other authors have proposed that AMPK (AMP-activated protein kinase) activation,which is a prospective target of cancer therapy,is involved in the apoptosis of colon carcinoma cells [74]. Magnolol demonstrated a number of pro-apoptotic functions,including DNA fragmentation,poly (ADP-ribose) polymerase and caspase-3 cleavage,and propidium iodide labeling. Magnolol was established to prevent the proliferation of Jurkat T leukemia cells and HL- 60 human cells by promoting apoptosis in a timeand dose- dependent manner. Caspase-2,-3 and -9 activation as well as the proteolytic cleavage of poly (ADP-ribose) polymerase were identified throughout apoptosis as encouraged by magnolol. Apoptotic signaling encouraged by magnolol is supported through the mitochondria via caspase-9,as the caspase downstream effectors are sequentially activated [75]. According to the previous studies,apoptosis generation is associated with cell cycle arrest at the G2/M phase,reducing the potential of the mitochondrial membrane,amplifying reactive oxygen species (ROS) generation,promoting apoptosis inducing factor (AIF) and cytochrome c (Cyto c) release from the mitochondria to the cytosol,down-regulating cyclin-dependent kinase 1 (CDK1),cyclin B1 and Bcl-2,and up-regulating p53,p21,and Bax. In addition,apoptosis induced by magnolol in MCF-7 cells through the mitochondrial AIF release and arrest pathway in the G2/M phase over the intrinsic pathway [76]. In the apoptosis death receptor pathway (extrinsic),ligands death receptor activation leads to caspase-8 activation. Caspase-8 activation can motivate caspase-3,which is an executioner caspase. Caspase- 3 activation can cleave PARP,resulting in apoptosis [77,78].
Shikonin
As a main component in Lithospermum erythrorhizon,a traditional Chinese herb,shikonin exhibits several biological roles,including anti-inflammatory,antitumor and antimicrobial effects. Results suggest that the shikonin amplified activation of ERK and ROS generation,also decreased Bcl2,which triggered apoptosis. It may also be a chemotherapeutic mediator in the treatment of osteosarcoma [79]. Shikonin promoted repressive effects in COLO 205 (colorectal cancer) and HL-60 leukemia human cells. In COLO 205 cells,apoptosis was induced by the presence of a sub-G1 DNA peak and DNA fragmentation,which was followed by damage to the mitochondrial membrane potential,cytochrome c release,the generation of reactive oxygen species (ROS),and pro-caspase-3 and -9 processing. Apoptotic cell death induced by shikonin was supplemented by Bad,p53,and p27 up-regulation as well as Bcl-X (L),and Bcl-2 down-regulation,although shikonin had little influence on Bax protein levels. Taken together,it has been recommended that apoptosis induced by shikonin is initiated by the release of cytochrome c into cytosol,caspase-3 activation,the processing of procaspase-9,PARP degradation,and DNA fragmentation,which is initiated by caspaseactivated deoxyribonuclease via DFF-45digestion [80]. Additionally,oxidative injury induced by shikonin activates apoptotic signaling cascades at a proximal point,motivates the JNK stress-related pathway and the release of cytochrome c,generates the dysfunction of the mitochondria,activates caspase,and signals apoptosis [81]. Shikonin encourages apoptotic death in cultured cancer cell lines by increasing intracellular ROS. Previous studies found that through the induction of ROS,shikonin encourages apoptosis in neuroblastoma cells [82]. Caspase-3,-7,and -9 were activated by treatment with shikonin in HLE B-3; its downstream target PARP was cleaved to become an activated form; cytochrome C was released from mitochondria. These results indicate that apoptosis was mediated through the mitochondrial intrinsic cell death pathway [83]. Bcl-2 and FLIP family members are essential regulators of the intrinsic [84],and extrinsic apoptotic pathways [85],respectively. Overall,published results recommend that both the extrinsic and the intrinsic apoptotic pathways may be involved in HCC apoptosis induced by shikonin [86].
Quercetin
As a nutritional flavonoid,quercetin exerts anticancer effects in some types of carcinomas by encouraging cell cycle arrest and stimulating apoptotic cell death. According to a previous study,quercetin encouraged apoptosis via the stimulation of caspase-9 and -3,but not caspase-8,in HepG2 cells. Quercetin enlarged Bax translocation and reduced the Bcl-xS:Bcl-xL ratio of the mitochondrial membrane. These data suggest that apoptosis by quercetin may take place via the direct induction of the caspase cascade (mitochondrial pathway) as well as by preventing survival signaling in HepG2 [87]. Based on the enlarged cell numbers in the sub-G1 phase,caspase-7 and caspase-3 proteolytic activation,the appearance of fragmented nuclei,the reduced potential of the mitochondrial membrane,caspase-9 and 3 proliferation and poly (ADP-ribose) polymerase protein degradation,was shown that U373MG cell death occurs via quercetin-induced apoptosis. Additionally,quercetin increased p53 expression through JNK activation and translocation to the mitochondria,which directed the release of cytochrome c from mitochondria to the cytosol [88]. The levels of PI3K,IGF-IR,Akt,p-Akt,Bad,cyclin D1,PARP,cytochrome c,and caspases-10 and -9 were evaluated to explore quercetin’s effect on insulinlike growth factor signaling as well as apoptosis in independent androgen PC-(3 prostate cancer cells). Quercetin expressively increased pro-apoptotic levels of Bad mRNA,cytochrome C,Bad protein,IGFBP-3,cleaved caspase-10 and -9 and PARP,and increased the activity of caspase-3 in PC-3 cells. Apoptosis was established in PC-3 cells treated with quercetin through damage to the mitochondrial membrane potential [89]. Quercetin influences both the intrinsic and extrinsic apoptotic pathways. In chronic lymphocytic leukemia (B-CLLs),was shown that quercetin promotes both fludarabine (intrinsic pathway),and DR- (extrinsic pathway) cell death [90]. In addition,previous experiments demonstrated that quercetin activates the mitochondria-dependent death pathway,in combination with TRAIL,as shown by the release of cytochrome c to the cytosol and Bid cleavage [91].
Resveratrol
Recent studies suggested that resveratrol (RV),which exists in red grape skins and other food products,is a natural plant polyphenol [92]. Resveratrol is a natural cardio-protective agent with anti-cancer properties. In vitro treatment by resveratrol causes mitogen-activated protein kinase (ERK1/2) nuclear translocation,consequesntial phosphorylation of Ser-15 of p53,and apoptosis. Intra-nuclear COX-2 increases p53 activation involved in the initiation of molecular stages in the ro-apoptotic actions of resveratrol in carcinoma cells. Thyroid hormone,estrogen,and epidermal growth factor influence ERK1/2 downstream to inhibit the apoptosis induced by resveratrol [93]. High efficacy of resveratrol induced apoptosis was shown in colo357 and capan-2 (pancreatic cancer cell lines). Caspase-3 activation was induced by resveratrol treatment in colo357 and capan-2,which are resveratrol-sensitive cells,and p21 and 053 were upregulated via resveratrol treatment [94]. Resveratrol encourages apoptotic cell death and prevents proliferation in various cancer cell types -in vitro- at higher non-physiological doses [95-97]. Additionally,resveratrol has been revealed to delay tumor growth and inhibit angiogenesis,in animal cancer models [98],to impede carcinogenesis [99,100],and to reduce experimental metastasis [101]. Resveratrol promoted apoptosis via caspase-independent AIF and downstream factors,and reduced the level of caspase-9 in the intrinsic apoptotic pathway in ASTC-a-1 cells (human lung adenocarcinoma) [92]. Although,caspase-8 activation did not occurs with resveratrol alone,co-treatment with both resveratrol and SB203580 not only improved FasL cleavage,but also promoted caspase-8,demonstrating that the extrinsic apoptotic pathway is possibly affected by this synergistic effect. SB203580 synergistically promotes apoptosis induced by resveratrol via the extrinsic pathway and facilitates the Bax-mediated intrinsic pathway [102].
Sulforaphane
Sulforaphane is a purified natural iso-thiocyanate found in cruciferous vegetables,and it has antileukemic properties in a wide range of ALL cell lines as well as pre-B-ALL and T-ALL primary lymphoblasts of pediatric patients. The treatment of -ALL leukemic cells with sulforaphane caused G2/M cell cycle arrest and apoptosis (dose-dependent),which was related to caspase-3,-8 and -9 activation,PARP inactivation,inhibition of the Cdc2/Cyclin B1 complex,and p53-independent upregulation of p21CIP1/WAF1. Sulforaphane repressed the mTOR and AKT survival pathways in the cell lines by reducing the levels of both phosphorylated and total proteins [103]. Treatment with sulforaphane inhibited cell growth,encouraged cell cycle blockade at G2-M,promoted oligonucleosomal DNA fragmentation,and amplified cyclin B1 expression in four human breast carcinoma cell lines: T47D,MDA-MB-468,MCF-7,and MDAMB- 231. The MDA-MB-231 cell apoptosis promoted by sulforaphane appeared to have originated via Fas ligand stimulation,which gives rise to the activation of poly (ADP-ribose) polymerase caspase-3,and caspase-8,whereas in the other breast carcinoma cell lines,apoptosis originated by reduced levels of Bcl- 2,cytochrome c release into the cytosol,activation of caspase-3 and caspase-9,but not caspase-8,and cleavage of poly (ADP-ribose) polymerase [104]. Published data has suggested that sulforaphane inhibits the activity of HDAC,activates apoptosis,and reduces the expression of key proteins in the proliferation of human breast carcinoma cells [104]. Treatment with sulforaphane encouraged apoptosis and cell viability in T24 cells in a concentrationdependent manner. Sulforaphane-induced apoptosis was associated with dysregulation of Bcl-2/Bax,cytochrome c release,and mitochondria dysfunction. The increased activity of caspase-9 and -3,but not caspase-8,was accompanied by the cleavage of poly ADP-ribose polymerase,indicating the involvement of the mitochondria-mediated intrinsic apoptotic pathway. These results demonstrate that sulforaphane has antitumor effects in bladder cancer cells through an ROS-mediated intrinsic apoptotic pathway,and suggest that ER stress and Nrf2 may represent strategic targets for sulforaphane-induced apoptosis [105]. The apoptosis encouraged by sulforaphane was associated with caspase-9,and -8 activation,the primary caspases of the intrinsic and extrinsic apoptotic pathways,respectively. It was also associated with cleavage of poly (ADP-ribose) polymerase and caspase-3 effector activation. Results suggest that sulforaphane induces mitotic arrest and apoptosis of 5637 cells via a ROSdependent pathway [106].
Acknowledgment
The authors would like to thank the University of Malaya for providing the research grant (PG053/2012B).
Competing Interests
The authors declare that no competing interest exists.
References
- Soheil Zorofchian Moghadamtousi,Hamed Karimian,Ramin Khanabdali,et al. (2014). Anticancer and Antitumor Potential of Fucoidan and Fucoxanthin,Two Main Metabolites Isolated from Brown Algae. The Scientific World Journal. 10: 768323.
- Reed JC. (2000). Mechanisms of apoptosis. Am. J. Pathol. 1415-1430.
- Danial NN,Korsmeyer SJ. (2004). Cell death: critical control points. Cell. 116: 205-219.
- Thompson CB. (1995). Apoptosis in the pathogenesis and treatment of disease. Science. 267: 1456–1462.
- Rowinsky EK. (2005). Targeted induction of apoptosis in cancer management: the emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J. Clin. Oncol. 23: 9394-9407.
- Call JA,Eckhardt SG,Camidge DR. (2008). Targeted manipulation of apoptosis in cancer treatment. Lancet Oncol. 9: 1002-1011.
- Iannolo G,Conticello C,Memeo L,De Maria R. (2008). Apoptosis in normal and cancer stem cells. Crit Rev Oncol Hematol. 66: 42–51.
- Burz C,Berindan-Neagoe I,Balacescu O,Irimie A. (2009). Apoptosis in cancer: key molecular signaling pathways and therapy targets. Acta Oncol. 48: 811–821.
- S Fulda,S Pervaiz. (2005). Apoptosis signaling in cancer stem cells. Int. J. Biochem. Cell Biol. 42: 31–38.
- IM Ghobrial,TE Witzig,AA Adjei. (2005). Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 55: 178–194.
- J Eberle,LF Fecker,T Forschner,et al. (2007). Apoptosis pathways as promising targets for skin cancer therapy. Br. J. Dermatol. 156: 18–24.
- DS Ziegler,AL Kung. (2008). Therapeutic targeting of apoptosis pathways in cancer. Curr. Opin. Oncol. 20: 97-103.
- EC Ledgerwood,IM Morison. (2009). Targeting the apoptosome for cancer therapy. Clin. Cancer Res. 15: 420–424.
- XK Lin,M Liu,CX Hu,et al. (2010). Targeting cellular proapoptotic molecules for developing anticancer agents from marine sources. Curr Drug Targets. 11: 708–715.
- K von Schwarzenberg,AM Vollmar. (2010). Targeting apoptosis pathways by natural compounds in cancer: marine compounds as lead structures and chemical tools for cancer therapy. Cancer Lett. 1007-1004.
- JC Reed. (2000). Mechanisms of apoptosis. Am. J. Pathol. 157: 1415–1430.
- L Oliver,FM Vallette. (2005). The role of caspases in cell death and differentiation. Drug Resist Updat. 8: 163–170.
- G Kroemer. (2003). Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun. 304: 433–435.
- S Gupta,GE Kass,E Szegezdi,B Joseph. (2009). The mitochondrial death pathway: a promising therapeutic target in diseases. J Cell Mol Med. 13: 1004–1033.
- K Abe,A Kurakin,M Mohseni-Maybodi,B Kay,R Khosravi-Far. (2000). The complexity of TNF-related apoptosis-inducing ligand. Ann N.Y Acad Sci. 926: 52–63.
- N Ozoren,WS El-Deiry (2003). Cell surface Death Receptor signaling in normal and cancer cells. Semin. Cancer Biol. 13: 135–147.
- ME Peter,PH Krammer. (2003). The CD95 (APO-1/Fas) DISC and beyond,Cell Death Differ. 10: 26–35.
- A Thorburn. (2004). Death receptor-induced cell killing. Cell Signal. 16: 139–144.
- SH Kaufmann,WC Earnshaw. (2000). Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 256: 42–49.
- N Zamzami,P Marchetti,M Castedo,et al. (1996). Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS Lett. 384: 53–57.
- GM Suarez-Jimenez,A Burgos-Hernandez,JM Ezquerra-Brauer. (2012). Bioactive peptides and depsipeptides with anticancer potential: sources from marine animals. Mar Drugs. 10: 963–986.
- LH Zheng,YJ Wang,J Sheng,et al. (2011). Antitumor peptides from marine organisms. Mar Drugs. 9: 1840–1859.
- Elmore S. (2007). Apoptosis: a review of programmed cell death. Toxicol Pathol. 35: 495-516.
- Lane D. (2004). p53 from pathway to therapy. Carcinogenesis. 25: 1077-1081.
- Fulda S,Debatin KM. (2004). Apoptosis signaling in tumor therapy. Ann N Y Acad Sci. 1028: 150-156.
- Ashkenazi A,Holland P,Eckhardt SG. (2008). Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/tumor necrosis factor–related apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol. 26: 3621-3630.
- Elmore S. (2007). Apoptosis: a review of programmed cell death. Toxicol Pathol. 35: 495-516.
- Bazzoni F,Beutler B. (1996). The tumor necrosis factor ligand and receptor families. N Engl J Med. 334: 1717-1725.
- Reddy BS,Wang CX,Samaha H,et al. (1997). Chemoprevention of colon carcinogenesis by dietary perillyl alcohol. Cancer Res. 57: 420–425.
- Hirano T,Abe K,Gotoh M,Oka K. (1995). Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. Br J Cancer. 72: 1380–1388.
- Jiang MC,Yang-Yen HF,Yen JJ,Lin JK. (1996). Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr Cancer. 26: 111–120.
- Chiao C,Carothers AM,Grunberger D,et al. (1995). Apoptosis and altered redox state induced by caffeic acid phenethyl ester (CAPE) in transformed rat fibroblast cells. Cancer Res. 55: 3576–3583.
- Fisher D A. (1996). Cell. 78: 539–547.
- Workman P. (1996). In Apoptosis and Cell Cycle Control in Cancer (ed. Thomas,N. S. B.). BIOS Scientific Publishers Ltd.,Oxford. 205–232.
- Lihong Wang,Liping Liu,Yan Shi,et al. (2012). Berberine Induces Caspase-Independent Cell Death in Colon Tumor Cells through Activation of Apoptosis-Inducing Factor. Plos one. 2012
- Novia KY Yip,W S Ho. (2013). Berberine induces apoptosis via the mitochondrial pathway in liver cancer cells. Oncology records. 1107-1112.
- Kotamballi N,Chidambara Murthy,Guddadarangavvanahally K,Jayaprakasha,Bhimanagouda S. (2012). The natural alkaloid berberine targets multiple pathways to induce cell death in cultured human colon cancer cells. European Journal of Pharmacology. 688: 14–21.
- Q Zhang,Z Qian,L Pan,H Li,H Zhu. (2012). Hypoxia-inducible factor 1 mediates the anti-apoptosis of berberine in neurons during hypoxia/ischemia. Acta Physiologica Hungarica. 99: 311–323.
- Choi YH,Han MH. (2011). Induction of Apoptosis by Combined-treatment with Genistein and TRAIL in U937 Human Leukemia Cells. AGRIS.
- Yong Maa,Jizhou Wanga,Lianxin Liua,et al. (2011). Genistein potentiates the effect of arsenic trioxide against human hepatocellular carcinoma: Role of Akt and nuclear factor-κB. Cancer Letters. 301: 75–84.
- Misty L,McDowella,Arabinda Dasa,et al. (2001). Neuroprotective effects of genistein in VSC4.1 motoneurons exposed to activated microglial cytokines. Neurochemistry International. 59: 175–184.
- Vanessa Ho¨rmann,James Kumi-Diaka,Marcia Durity,Appu Rathinavelu. (2012). Anticancer activities of genistein-topotecan combination in prostate cancer cells. J Cell Mol Med. 16: 2631-2636.
- Hye SS,Han SC,Hyeong SC,et al. (2011). Phytoestrogens Induce Apoptosis via Extrinsic Pathway,Inhibiting Nuclear Factor-κB Signaling in HER2-overexpressing Breast Cancer Cells. Anticancer Research. 31: 3301-3314.
- Kyriaki Zikaki,Ioanna-Katerina Aggeli,Catherine Gaitanaki,Isidoros Beis. (2014). Curcumin induces the apoptotic intrinsic pathway via upregulation of reactive oxygen species and JNKs in H9c2 cardiac myoblasts. Apoptosis. 19: 958-974.
- Min-Liang,Tze-Sing Huangb,Jen-Kun Linc. (1996). Curcumin,an antioxidant and anti-tumor promoter,induces apoptosis in human leukemia cells. 1317: 95–100.
- Shin-Hw AW,Liang WH,Jai SY,et al. (2010). Curcumin Induces Apoptosis in Human Non-small Cell Lung Cancer NCI-H460 Cells through ER Stress and Caspase Cascade- and Mitochondria-dependent Pathways. Anticancer Research. 30: 2125-2133.
- Jian Zhang,Yongping Du,Changgui Wu,et al. (2010). Curcumin promotes apoptosis in human lung adenocarcinoma cells through miR-186* signaling pathway. Oncology reports. 24: 1217-1223.
- Chu-Wen Yang,Chi-Lun Chang,Hsin-Chen Lee,et al. (2012). Curcumin induces the apoptosis of human monocytic leukemia THP-1 cells via the activation of JNK/ERK Pathways. BMC Complementary and Alternative Medicine. 12: 22.
- McGee,Harold. (2004). A survey of tropical spices,McGee on Food and Cooking. Hodder and Stoughton. 426.
- Poltronieri Juliana,B Becceneri Amanda,M Fuzer Angelina,et al. (2014). [6]-gingerol as a Cancer Chemopreventive Agent: A Review of Its Activity on Different Steps of the Metastatic Process. Mini Reviews in Medicinal Chemistry. 14: 313-321.
- Wang CC,Chen LG,Lee LT,et al. (2003). Effects of 6-gingerol,an antioxidant from ginger,on inducing apoptosis in human leukemic HL-60 cells. In Vivo. 17: 641-645.
- Yang G,Wang S,Zhong L,et al. (2012). 6-Gingerol Induces Apoptosis through Lysosomal-Mitochondrial Axis in Human Hepatoma G2 Cells. Phytother. Res. 26: 1667–1673.
- Kim HW,Oh DH,Jung C,Kwon DD,Lim YC. (2011). Apoptotic Effects of 6-Gingerol in LNCaP Human Prostate Cancer Cells. Soonchunhyang Med Sci. 17: 75-79.
- Vijay Mohan,Dhanya Nambiar,Raosaheb K Kale,Rana P Singh. (2013). Cell-Death—Inducing Mechanisms of Cancer Chemopreventive Agents. Mitochondria as Targets for Phytochemicals. Cancer Prevention and Therapy. 61-84.
- Hyun-Woo Kim,Deuk-Hee Oh,Chaeyong Jung,et al. (2011). Apoptotic Effects of 6-Gingerol in LNCaP Human Prostate Cancer Cells. Soonchunhyang Medical Science. 17: 75-79.
- Yuan J,Horvitz HR. (2004). A first insight into the molecular mechanisms of apoptosis. Cell. 116: 53-6.
- Utz PJ,Anderson P. (2000). Life and death decisions: regulation of apoptosis by proteolysis of signaling molecules. Cell Death Differ. 7: 589-602.
- Ishiguro K,Ando T,Maeda O,et al. (2007). Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms. Biochem Biophys Res Commun. 362: 218-23.
- Lee SH,Cekanova M,Baek SJ. (2008). Multiple mechanisms are involved in 6- gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol Carcinog. 47: 197-208.
- Stennicke HR,Jurgensmeier JM,Shin H,et al. (1998). Pro-caspase-3 is a major physiologic target of caspase- 8. J. Biol. Chem. 273: 27084–27090.
- Methot N,Huang J,Coulombe N,et al. (2004). Differential efficacy of caspase inhibitors on apoptosis markers during sepsis in rats and implication for fractional inhibition requirements for therapeutics. J. Exp. Med. 199: 199–207.
- Fazekas Z,Gao D,Saladi RN,et al. (2003). Protective effects of lycopene against ultraviolet B-induced photodamage. Nutr and Cancer. 47: 181-187.
- Venier NA,Colquhoun AJ,Fleshner NE,Klotz LH,Venkateswaran V. (2012). Lycopene enhances the anti-proliferative and pro-apoptotic effects of capsaicin in prostate cancer in vitro. Journal of Cancer Therapeutics and Research. 1: 3.
- Rongchuan Yue,Houxiang Hu mail,Kai Hang Yiu,et al. (2012). Lycopene Protects against Hypoxia/Reoxygenation-Induced Apoptosis by Preventing Mitochondrial Dysfunction in Primary Neonatal Mouse Cardiomyocytes. PloS ONE. 7: e50778.
- Di Mascio P,Kaiser S,Sies H. (1989). Lycopene as the most efficient biological carotenoid singlet oxygen quamcher. Arch Biochem Biophys. 274: 532-8.
- Russo M,Spaguolo C,Tedesco I,Russo GL. (2010). Phytochemicals in cancer prevention and therapy: truth or dare? Toxins. 2: 517-51.
- Cristina Trejo-Solís,Jose Pedraza-Chaverrí,Mónica Torres-Ramos,et al. (2013). Multiple Molecular and Cellular Mechanisms of Action of Lycopene in Cancer Inhibition. Evidence-Based Complementary and Alternative Medicine. 17.
- Tsai JR,Chong IW,Chen YH,et al. (2014). Magnolol induces apoptosis via caspase-independent pathways in non-small cell lung cancer cells. Arch Pharm Res. 37: 548-57.
- Park JB,Lee MS,Cha EY,et al. (2012). Magnolol-induced apoptosis in HCT-116 colon cancer cells is associated with the AMP-activated protein kinase signaling pathway. Biol Pharm Bull. 35: 1614-20.
- Chi,chis. (2012). Cell. 1213.
- Zhou Y,Bi Y,Yang C,et al. (2013). Magnolol induces apoptosis in MCF-7 human breast cancer cells through G2/M phase arrest and caspase-independent pathway. Pharmazie. 68: 755-62.
- Tewari M,Quan LT,O’Rourke K,et al. (1995). Yama/CPP3213,a Mammalian Homolog of CED-3,Is a CrmA-Inhibitable Protease That Cleaves the Death Substrate Poly (ADP-Ribose) Polymerase. Cell. 81: 801-809.
- Ghobrial IM,Witzig TE,Adjei AA. (2005). Targeting Apoptosis Pathways in Cancer Therapy. CA Cancer J Clin. 55: 178-194.
- Chang IC,Huang YJ,Chiang TI,Yeh CW,Hsu LS. (2010). Shikonin induces apoptosis through reactive oxygen species/extracellular signal-regulated kinase pathway in osteosarcoma cells. Biol Pharm Bull. 33: 816-24.
- Hsu PC,Huang YT,Tsai ML,et al. (2004). Induction of apoptosis by shikonin through coordinative modulation of the Bcl-2 family,p27,and p53,release of cytochrome c,and sequential activation of caspases in human colorectal carcinoma cells. J Agric Food Chem. 52: 6330-7.
- Mao X,Yu CR,Li WH,et al. (2008). Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res. 18: 879-88.
- Yang J-T,Li Z-L,Wu J-Y,et al. (2014). An Oxidative Stress Mechanism of Shikonin in Human Glioma Cells. PLoS ONE. 9: e94180.
- Wan-Rong Huang,Yue Zhang,Xin Tang. (2014). Shikonin Inhibits the Proliferation of Human Lens Epithelial Cells by Inducing Apoptosis through ROS and Caspase-Dependent Pathway. Molecules. 19: 7785-7797.
- Youle,RJ. (2008). Strasser,A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9: 47–59.
- Kim Y,Suh N,Sporn M,Reed JC. (2002). An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J. Biol. Chem. 277: 22320–22329.
- Ke Gong,Wenhua Li Shikonin. (2011). a Chinese plant-derived naphthoquinone,induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: A potential new treatment for hepatocellular carcinoma. Free Radical Biology & Medicine. 51: 2259–2271.
- Granado-Serrano AB,Martín MA,Bravo L,Goya L,Ramos S. (2006). Quercetin induces apoptosis via caspase activation,regulation of Bcl-2,and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J Nutr. 136: 2715-21.
- Hyeonji Kim,Jeong Yong Moon,Kwang Seok Ahn,Somi Kim Cho. (2013). Quercetin Induces Mitochondrial Mediated Apoptosis and Protective Autophagy in Human Glioblastoma U373MG Cells. Oxidative Medicine and Cellular Longevity. 10.
- Senthilkumar K,Elumalai P,Arunkumar R,et al. (2010). Quercetin regulates insulin like growth factor signaling and induces intrinsic and extrinsic pathway mediated apoptosis in androgen independent prostate cancer cells (PC-3). Mol Cell Biochem. 344: 173-84.
- M Russo,C Spagnuolo,S Volpe,et al. (2010). Quercetin induced apoptosis in association with death receptors and fludarabine in cells isolated from chronic lymphocytic leukaemia patients. British Journal of Cancer. 103: 642–648.
- Faiy H. Psahoulia,Konstantinos G Drosopoulos,Lenka Doubravska,Ladislav Andera,Alexander Pintzas. (2007). Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther. 6: 2591–9.
- Lin HY,Tang HY,Davis FB,Davis PJ. (2011). Resveratrol and apoptosis. Ann N Y Acad Sci. 1215: 79-88.
- Zhang W,Wang X,Chen T. Resveratrol induces apoptosis via a Bak-mediated intrinsic pathway in human lung adenocarcinoma cells. Cell Signal. 24: 1037-46.
- Zhou JH,Cheng HY,Yu ZQ,et al. (2011). Resveratrol induces apoptosis in pancreatic cancer cells. Chin Med J (Engl). 124: 1695-9.
- Hsieh TC,Wu JM. (1999). Differential effects on growth,cell cycle arrest,and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp Cell Res. 249: 109-115.
- Pozo-Guisado E,Alvarez-Barrientos A,Mulero-Navarro S,et al. (2002). The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochem Pharmacol. 64: 1375-1386.
- Kim YA,Choi BT,Lee YT,et al. (2004). Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol Rep. 11: 441-446.
- Tseng SH,Lin SM,Chen JC,et al. (2004). Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin Cancer Res. 10: 2190-2202.
- Jang M,Cai L,Udeani GO,et al. (1997). Cancer chemopreventive activity of resveratrol,a natural product derived from grapes. Science. 275: 218-220.
- Gusman J,Malonne H,Atassi G. (2001). A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 22: 1111-1117.
- Busquets S,Ametller E,Fuster G,et al. (2006). Resveratrol,a natural diphenol,reduces metastatic growth in an experimental cancer model. Cancer Lett.
- Li H,Wang X,Chen T,Qu J. (2012). p38 inhibitor SB203580 sensitizes the resveratrol-induced apoptosis in human lung adenocarcinoma (A549) cells. J Biochem Mol Toxicol. 26: 251-7.
- Suppipat K,Park CS,Shen Y,Zhu X,Lacorazza. (2012). Sulforaphane Induces Cell Cycle Arrest and Apoptosis in Acute Lymphoblastic Leukemia Cells. PLoS ONE. 7: e51251.
- Allison Pledgie-Tracy,Michele D Sobolewski,Nancy E Davidson. (2007). Sulforaphane induces cell type–specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 6: 1013-1021.
- Jo GH,Kim GY,Kim WJ,Park KY,Choi YH. (2014). Sulforaphane induces apoptosis in T24 human urinary bladder cancer cells through a reactive oxygen species-mediated mitochondrial pathway: The involvement of endoplasmic reticulum stress and the Nrf2 signaling pathway. Int J Oncol.
- Park HS,Han MH,Kim GY,et al. (2014). Sulforaphane induces reactive oxygen species-mediated mitotic arrest and subsequent apoptosis in human bladder cancer 5637 cells. Food Chem Toxicol. 64: 157-65.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences