Ganoderma lucidum Polysaccharides Extraction, yields and its Biological Applications
Yugandhar Parepalli, Murthy Chavali*, Sudhakar Reddy Pamanji, Meenakshi Singh
Yugandhar Parepalli1, Murthy Chavali2,3*, Sudhakar Reddy Pamanji4, Meenakshi Singh5
1Department of Sciences and Humanities,Division of Chemistry, VFSTRU, Guntur 52221, Andhra Pradesh, INDIA
2Department of Chemistry, Shree Velagapudi Rama Krishna Memorial College (SVRMC), Nagaram 522268 Guntur District, Andhra Pradesh, INDIA
3Department of Chemistry, NTRC, MCETRC, Chinnaravuru, Tenali 5222, Guntur District, Andhra Pradesh, INDIA
4Department of Zoology, Vikrama Simhapuri University Post-Graduate Centre, Kavali 52420, Andhra Pradesh, INDIA
5Department of Botany, Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara 39000, Gujarat, INDIA
*Corresponding Author:
Murthy Chavali
Department of Chemistry
Shree Velagapudi Rama Krishna Memorial College (SVRMC)
Nagaram 522268 Guntur District, Andhra Pradesh, INDIA
Tel: +91-8309-337-736
E-mail: ChavaliM@outlook.com
Received: June 01, 2020; Accepted: August 10, 2020; Published: August 17, 2020
Citation: Parepalli Y, Chavali M, Pamanji SR, et al. Ganoderma lucidum Polysaccharides Extraction, yields and its Biological Applications. Electronic J Biol, 16:4.
Abstract
The Reishi mushroom, Ganoderma lucidum is an edible herbal home remedy to boost the immune system, especially in the Asian countries. Its fruiting body can thrive well in a hot and humid climate and contain specific bioactive macromolecules like triterpenoids, phenolic compounds, steroids, nucleotides and their derivatives polysaccharides and glycoproteins which have strong therapeutic properties. In this mini-review, the focus is on medicinal G. lucidum polysaccharides, one of the effective constituents as a health-promoting agent and its methods of extraction and purification to reflect the current status of characterization techniques in clinical practices. An overview of conformational properties, different analytical techniques and other methods involved were briefly discussed. A detailed account of significant biological applications of G. lucidum polysaccharides like antitumor, antiinflammatory, antiviral and anticancer activities was tabulated and discussed
Introduction
Mushrooms have been known for their edible, medicinal resources and antitumor substances for many years. The fungi belonging to the genus Ganoderma are popular medicinal mushrooms, widely used in China, Japan and Korea over the past two millennia [1,2]. The most frequently cited Ganoderma species used in research publications on the cultivation, chemical analysis, pharmacology and medicinal effects is the Ganoderma lucidum (G. lucidum), an edible medicinal mushroom commonly known as Reishi or Manentake (Japanese) or Lingzhi (Chinese) [3]. The incredible curative properties have won it the titles of ‘supernatural mushroom’, ‘magic mushroom’ and ‘plant of longevity or immortality’, produced not only in its native East Asian countries such as China, India, Japan, Korea, Taiwan, and Malaysia but also in the USA. G. lucidum has been reported to have many pharmacological effects including immune-modulating, anti-atherosclerotic, anti-inflammatory, analgesic, chemopreventive, anti-tumour, radioprotective, sleep-promoting, antibacterial, antiviral (including anti-HIV), hypolipidemic, anti-fibrotic, hepatoprotective, diabetic, antioxidative and radical-scavenging, antiaging, hypoglycemic, and antiulcer properties [4- 10]. Reishi mushroom has now become recognized as an alternative adjuvant in the treatment of leukaemia, carcinoma, hepatitis, and diabetes.
Polysaccharides, triterpenes, sterols and peptidoglycans are the major chemical constituents of Ganoderma lucidum, along with oleic acid, soluble proteins, amino acids, ergosterol peroxide (5,8-epidioxy-ergosta-6,22E-dien-3-ol), and the cerebrosides (4E’,8E)-N-D-2’–hydroxylstearoyl-1-O- β-D-glucopyranosyl-9-methyl-4-8-sphingadienine, and (4E’,8E)-N-D-2’-hydroxypamitoyl-1-O-β-Dglucopyranosyl- 9-methyl-4-8-sphingadienine and cyclo-octasulfur along with inorganic ions like Iron, Manganese, Germanium, Magnesium, Zinc, Copper, and Calcium [11-13]. The fruiting body of the Ganoderma lucidum is shown in Figure 1. G. lucidum spore cell wall contains a high amount of polysaccharides, which are natural macromolecular compounds with complex and versatile biological activities. The spores also contain choline, betaine, tetracosanoic acid, stearic acid, palmitic acid, ergosta-7, 2, 2-dien-3-ol, nonadecanoic acid, behenic acid, tetracosane, hentriacontane, ergosterol, and β-sitosterol. One of the lipids isolated from G. lucidum is pyrophosphatidic acid [14,15].

Figure 1: Ganoderma lucidum (Reishi or Lingzhi).
In recent years, polysaccharides extracted from G. lucidum have been regarded as an important class of anticoagulants, immunomodulating and antitumour with antioxidant activities, antiproliferative activities, antiviral and antiprotozoal activities [16- 20]. G. lucidum polysaccharides such as β-Dglucans, heteropolysaccharides, and glycoprotein have been isolated and characterized; considered as the major contributors of bioactivity of the mushroom. β-D-glucans consist of a linear backbone of β-(1→3)- linked D-glucopyranosyl groups with varying degrees of branching from the C6 position. In addition to water-soluble β-D-glucans, β-D-glucans co-exist with hetero-polysaccharide chains of xylose, mannose, galactose, uronic acid and β-D-glucans–protein complexes that are present at 10–50% in dry G. lucidum, presence of various reactive groups in their structure, polysaccharides can be easily modified chemically and biochemically. Moreover, the presence of hydrophilic groups in their structure, such as hydroxyl, carboxyl and amino groups, enhance bio-adhesion with biological tissues, like epithelia and mucous membranes, forming non-covalent bonds, a useful strategy to improve the bioavailability of drugs included in drug delivery systems.
Extraction of polysaccharides
Polysaccharides extraction is fairly time consuming and slow process, the literature suggests several published articles using different approaches for the extraction of polysaccharides from the spores of G. lucidum. The most common approaches were hot water extraction (HWE) for the extraction of watersoluble polysaccharides and alkaline extraction is used for the extraction of water-insoluble polysaccharides.
Traditional use of hot water extraction (HWE) was the cause for a lower yield, longer extraction times and high-temperature process. To get the better yields other techniques like Ultrasound Microwave- Assisted Extraction (UMAE) by Sheng et al, Ultrasonic Assisted Extraction (UAE) by Liyan et al, [23] breaking the spores of the fungus G. lucidum by supercritical CO2 by Yu-Jie et al, breaking the spores of G. lucidum by fermentation with Lactobacillus plantarum by Chaiyavat, Chakkrapong and Sasithorn [24] and alkaline extraction of polysaccharides (AEP) by Gao et al, [25,27] were reported. The percentage yields of different techniques were given in Table 1. The most effective procedure of AEP results showed optimized yields from the fruiting body of G. lucidum of 6.81% under alkaline extraction conditions.
Breaking spores
by Supercritical CO22.98SolubleYu-Jie et al. [28]5Breaking spores by fermentation using Lactobacillus plantarumNANAChaiyavat, Chakkrapong and Sasithorn. [29]6UMAE3.9SolubleSheng and Zheng [30]7UAE2.07SolubleLiyan et al. [31]8AEP8.21In-solubleSheng et al. [32]9AEP1.41In-solubleJinghua et al. [33]10AEP6.81In-solubleGao et al. [34]
HWE: Hot Water Extraction; UMAE; Ultrasound Microwave Assisted Extraction; UAE: Ultrasonic Assisted Extraction; AEP: Alkaline Extraction of polysaccharides; NA: Not Available
Table 1. The percentage yield of polysaccharides extracted from Ganoderma lucidum using different
Extraction Procedure
The dried sample was grounded into a fine powder and defatted with petroleum ether, ethyl acetate and methanol. Then mixed with 80% ethanol and shaken at 30°C for 24 h, to remove most of the polyphenols and monosaccharides. Water-soluble polysaccharides were extracted stepwise with a 0.2 M phosphate buffer solution (PBS) of pH-7 at 25°C, 80°C and 120°C. In each step, the PBS suspension was centrifuged and to the supernatant was added a large quantity of ethanol to precipitate the polysaccharide. The precipitates of G. lucidum polysaccharide (GLP) were designated GLP I, GLP II and GLP III in the order of increasing extraction temperature.
The residue obtained from the last PBS suspension was then treated with 1% ammonium oxalate and acetic acid was added to the supernatant to precipitate the water-insoluble polysaccharides and was designated as GLPIV the residue obtained was treated with 5% NaOH at 25°C, acetic acid was added to precipitate the polymers and designated as GLPV. Ethanol was added to the supernatant to get the final polysaccharides GLPVI. Crude polysaccharides of G. lucidum at different stages of extraction were obtained. These extraction procedures were similar to those reported by Sone et al, [28-35] for Reishi mushroom and by Wang et al, [36] for G. tsuage mushroom. The procedure for the complete extraction process of polysaccharides from G. lucidum is depicted in Figure 2.
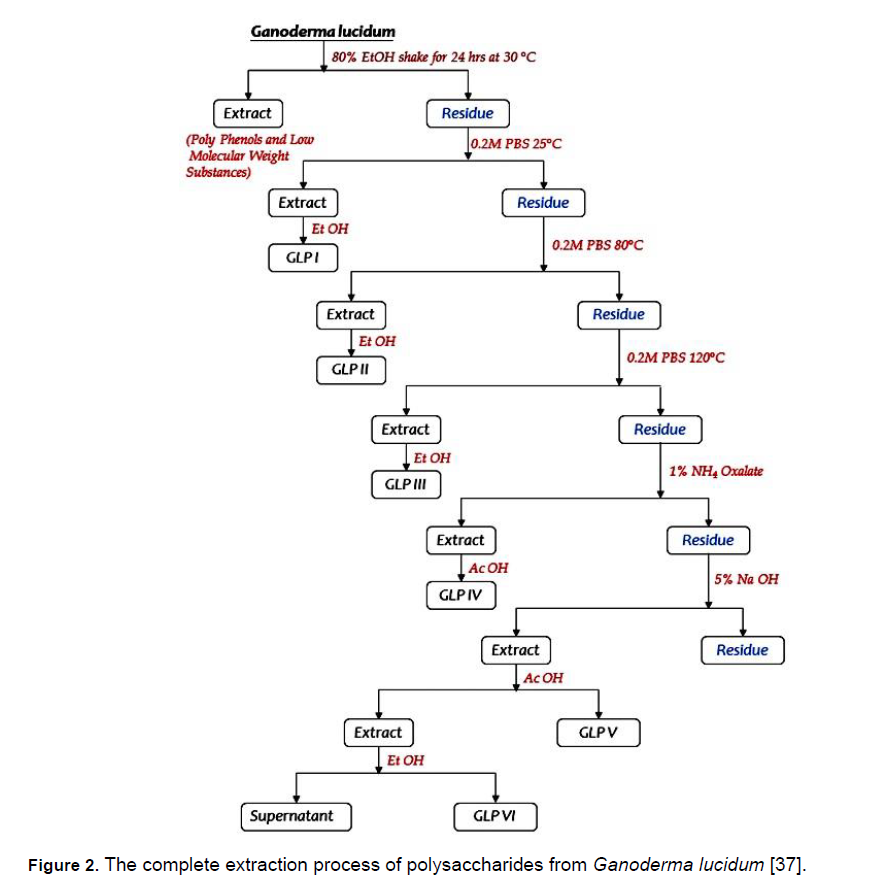
Figure 2: The complete extraction process of polysaccharides from Ganoderma lucidum [37].
Purification of Polysaccharides
Extracted polysaccharides were purified by a combination of techniques, such as ethanol precipitation, fractional precipitation, and acidic precipitation with acetic acid, ion-exchange chromatography, gel filtration, and affinity chromatography. The ethanol precipitation excludes the impurities from the polysaccharides. The separation of acidic and neutral polysaccharides can be achieved by anion-exchange chromatography on diethyl-amino-ethanol cellulose (DEAE-C) column. The neutral polysaccharide in the mixture is first eluted by an appropriate running buffer; the acidic polysaccharide is then eluted at a higher salt concentration.
Neutral polysaccharides later separated into α-glucans (adsorbed fraction) and β-glucans (nonadsorbed fraction) with the help of gel filtration and affinity chromatography. This process now allows for the highly specific and efficient purification of some carbohydrates. The complete purification process of G. lucidum polysaccharides is given in Figure 3.
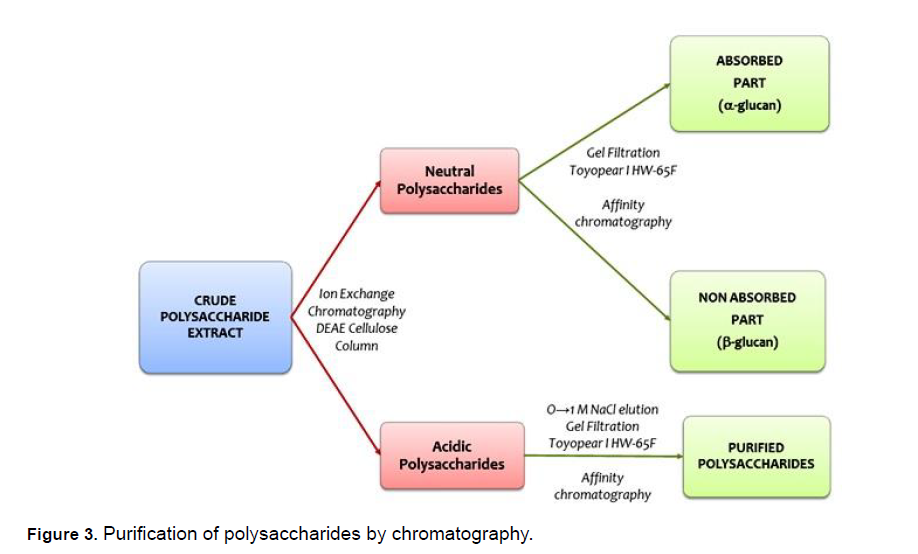
Figure 3: Purification of polysaccharides by chromatography.
Conformational properties and analytical techniques
Polysaccharides having hyper-branched structures, to characterize such structures for their chemical structure and chain conformations are not an easy task. The chemical structures were analyzed by FTIR spectroscopy, Raman spectroscopy, NMR spectroscopy- Liquid-state NMR (1D and 2D) and Solid-state NMR, several chromatographic techniques like Gas Chromatography (GC), GC– Mass (GC–MS) and High-Performance Liquid Chromatography (HPLC) were employed for fractionation of polysaccharides. Chain conformations of polysaccharides in solutions were investigated using static and dynamic light scattering, viscosity analysis based on the theory of dilute polymer solutions, and Atomic Force Microscopy (AFM) including single molecular AFM and AFM-based single-molecule Force Spectroscopy, fluorescence correlation spectroscopy and NMR spectroscopy.
Characterization of Polysaccharides
The chemical structures of polysaccharides, such as the sugar composition, type of glycosyl linkage and the branched structures, were characterized by spectral analysis, chemical analysis and chromatography.
FTIR spectroscopy
FTIR spectroscopy technique was used in investigating the vibrations of molecules and polar bonds between the different atoms. Fourier transform infrared (FT-IR) spectroscopy is a physicochemical method based on measurement of vibration of a molecule excited by IR radiation at a specific wavelength range. Functional groups present in a molecule tend to absorb IR radiation in the same wavenumber range regardless of other structures in the molecule, and spectral peaks are derived from the absorption of bond vibrational energy changes in the IR region. Structures of polysaccharides, such as monosaccharide types, glucosidic bonds and functional groups, can be analyzed using FTIR spectroscopy [37,38]. In the range of 1100–1010 cm-1, three strong absorption peaks appear for pyranoside, and two peaks for furanoside.
Raman spectroscopy
Compared with FTIR spectroscopy, Raman spectroscopy is highly sensitive to detect the vibrations of molecules and non-polar bonds of the same atom. Raman spectroscopy is the best suitable technique to characterize the helical conformation and the plane fold of bio-macromolecules [39]. The Raman spectra of saccharides segregated into four regions: the bands in the range of 350-600 cm-1 are assigned to skeletal modes of pyranose rings; the anomeric region is from 600 to 950 cm-1, the glycosidase stretching modes appear in the region 950–1200 cm-1; and the CH2 and C–OH deformations region is from 1200 to 1500 cm-1.
NMR spectroscopy
NMR spectroscopy has become the most powerful and non-invasive physicochemical technique for determining polysaccharide structures providing detailed structural information of polysaccharides, including identiïìÃÂcation of monosaccharide composition, elucidation of α- or β-anomeric conïìÃÂgurations, the establishment of linkage patterns, and sequences of the sugar units in polysaccharides.
Liquid-state NMR
The liquid-state NMR has become recognized as an important developing tool for chemical structural analysis of polysaccharides [40]. Most polysaccharides can be dissolved in water and dimethyl sulfoxide (DMSO), thus denatured water and DMSO (D2O and DMSO-d6) are common solvents for polysaccharides in the liquid-state NMR experiments. The proton signals of polysaccharides overlap in the range of 3.5-5.5 ppm in the 1H NMR spectrum, it is difficult to assign them. Leeuwen et al, [41], investigated the 1H NMR spectroscopy of the primary structural characterization of α-D-glucans in detail, in which chemical shift patterns for (α1→2)- , (α1→3)-, (α1→4)- and (α1→6)-linked D-glucose residues were analyzed. In contrast, the range of 13C chemical shifts of polysaccharides is much wider than that of 1H chemical shift, which comes from 60 to 110 ppm.
Solid-state NMR
Solid-state NMR in contrast with liquid-state NMR the line widths become broader mainly due to the anisotropic character and dipolar interaction [42]. The anisotropic parts of the interactions from the molecules can be removed when the solid sample rotates at 54.7°. Magic-angle-spinning (MAS) is essential to achieve high-resolution 13C solid-state NMR spectra [43]. The intensity of the solid 13C signals can be enhanced using cross-polarization (CP) technology, in which the polarization transfers from 1H to 13C. In recent years, solid-state NMR is used to analyze the chemical structures of polysaccharide to overcome the solubility problem, since the samples can be measured in a solid and dehydrated form. Spevacek and Brus, Pizzoferrato et al, [44,45], have reported the ratio between proteins and polysaccharides was directly determined through solid 13C CP/MAS spectroscopy.
Chromatography
The monosaccharide compositions, types of glycosidic linkages and branching of polysaccharides may be also analyzed by chromatography. GC, GC–MS and HPLC methods are employed after polysaccharides are hydrolysed by trifluoroacetic acid (TFA) or derived by the methylation, periodic acid oxidation and Smith degradation [46-49].
Chain Conformational analysis of Polysaccharides in Solution
Conformation of polysaccharides in solutions; especially in aqueous solutions, can be investigated according to the theory of dilute polymer solutions. The intrinsic viscosity η is a characteristic property of polysaccharide solution. Huggins and Kraemer's equations are used to estimate the η value by extrapolating to infinite dilution [50-52].
ηsp/C= η +K'η2C
(lnηr)/C= η +K"η2C
Where K' is the Huggins constant and K" is the Kraemer constant, ηsp/C is the reduced specific viscosity, and (ln ηr)/C is the inherent viscosity.
Other Methods
The AFM-based single-molecule force spectroscopy (AFM–SMFS) technology is a powerful tool to characterize the force-induced conformational transitions, the dynamics, and supramolecular structures of polysaccharides at the molecular level [53-58].
Fluorescence correlation spectroscopy (FCS) is interesting to determine the conformations and sizes of polysaccharides at a lower concentration of about10-8mol/l [59].
Biological Applications
Ganoderma lucidum has been used to treat various human diseases such as allergy, arthritis, bronchitis, gastric-ulcer, hyperglycemia, hypertension, chronic hepatitis, hepatopathy, insomnia, nephritis, neurasthenia, scleroderma, inflammation, and cancer. The fruiting bodies or spores of G. lucidum were linked to possible therapeutic effects (Table 2). The mechanisms of action involve the gut microbiota, meaning the polysaccharides act as prebiotics in the digestive system [60], Different compounds with various biological activities were extracted from mycelia. Current biological/biomedical applications of G. lucidum were given in Figure 4 [61-65].
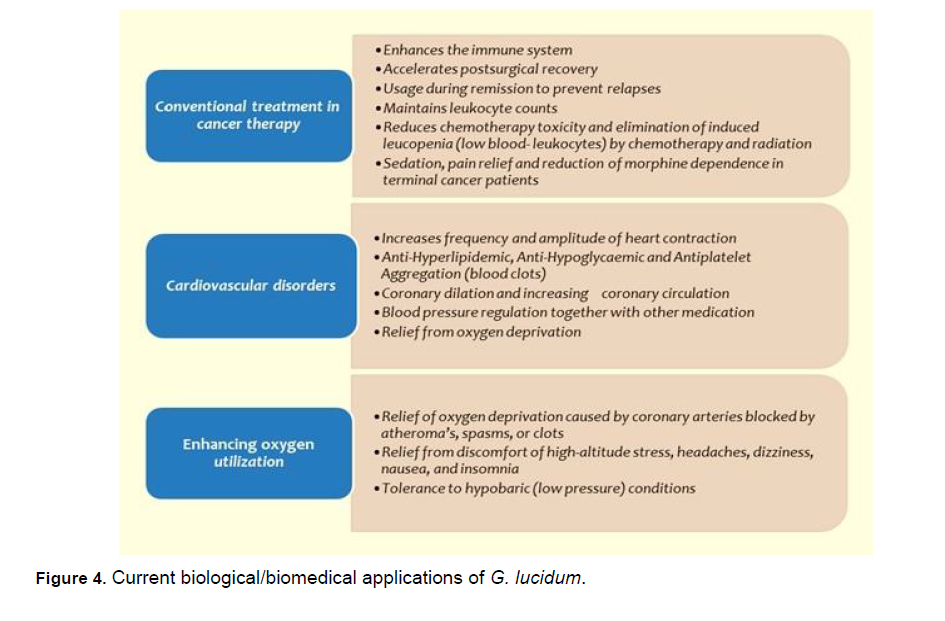
Figure 4: Current biological/biomedical applications of G. lucidum.
(95% polysaccharides and 5% peptides)Activation of the immune response, stimulation of the IL-1β, IL-6, TNF-α, and IFN-γ production by macrophages and T lymphocytes, Inhibition of neutrophil apoptosis, Induction of neutrophil phagocytosis, Induction of GSTWang et al. [67]
Hsu, Lee and Lin [68]
Hsu et al. [69]
Kim, Kacewand Lee [70]3G009, amino polysaccharidesAntioxidantLee et al. [71]4Glycoproteins (with fucose)Stimulation of IL-1, IL-2 and IFN-γ expression in spleen cellsWang et al. [72]5GLIS, proteoglycansActivation of b-lymphocytesZhang et al. [73]6CerebrosidesInhibition of DNA-polymeraseMizushina et al. [74]TRITERPENES7Ganoderic acid (U, V, W, X, Y)Cytotoxic for hepatoma cellsShiao et al. [75]8Ganoderic acid (A, C)Inhibition of farnesyl protein transferaseToth, Luu and Ourission [76]9Lucidimol (A, B), Ganodermanondiol, Ganoderiol F, GanodermanontriolCytotoxic for sarcoma and lung carcinoma cellsMin et al. [77]
El-Mekkawy et al. [78]
Min et al. [79]10Ganoderic acid FInhibition of angiogenesisKimura, Taniguchi and Baba [80]11PhenolsAntioxidantMau, Lin and Chen [81]12LipidsGrowth inhibition of hepatoma, sarcoma S-180 and reticulocyte sarcoma L-II in vivoLiu et al. [82]
Table 2. Biologically active components in Ganoderma lucidum
Polysaccharides of G. lucidum have been used for a broad spectrum of health benefits from preventative measures and maintenance of health to the regulation or treatment of chronic as well as acute life-threatening illness. Nowadays more research is focussed on bioactive molecules from G. lucidum including polysaccharide as a chemotherapeutic agent to treat cancer [83]. In China, clinical trials on the approved drug are undergoing on G. lucidum polysaccharides to treat myopathy and other diseases [84]. Some of the significant biological/ biomedical applications of this mushroom were given in Table 3.
Players on "living high-training low"Zhang et al. [102]19Effects of Ganoderma lucidum spores on HepG2 cells proliferation and growth cycleLi et al. [103]20A randomized clinical trial of an ethanol extract of Ganoderma lucidum in men with lower urinary tract symptomsNoguchi et al.[104]21Serum amyloid A mediates the inhibitory effect of Ganoderma lucidum polysaccharides on tumour cell adhesion to endothelial cellsYing et al. [105]22The dual roles of Ganoderma antioxidants on urothelial cell DNA under carcinogenic attackYuen and Gohel [106]23Ganoderma lucidum polysaccharides can induce human monocytic leukaemia cells into dendritic cells with immuno-stimulatory functionWing et al.[107]24Effect of an extract of Ganodermalucidum in men with lower urinary tract symptoms: a double-blind, placebo-controlled randomized and dose-ranging studyNoguchi et al.[108]25Telomerase-associated apoptotic events by mushroom Ganoderma lucidum on premalignant human urothelial cellsYuen, Goheland Au [109]26Ganoderma lucidum polysaccharides in human monocytic leukaemia cells: from gene expression to network constructionKun et al.[110]27Herbal mixtures containing the mushroom G. lucidum improve recovery time in patients with herpes genitalis and labialisHijikata, Yamada and Yasuhara [111]28Androgen receptor-dependent and -independent mechanisms mediate Ganoderma lucidum activities in LNCaP prostate cancer cellsZaidman et al. [112]29Ganoderma lucidum polysaccharides enhance CD14 endocytosis of LPS and promote TLR4 signal transduction of cytokine expressionHua et al.[113]30The potential of a novel polysaccharide preparation (GLPP) from Anhui-grown Ganoderma lucidum in tumour treatment and immunostimulationPang et al.[114]31Ganoderma lucidum polysaccharide peptide reduced the production of proinflammatory cytokines in activated rheumatoid synovial fibroblastHo et al.[115]32Inhibition of oxidative stress-induced invasiveness of cancer cells by Ganoderma lucidum is mediated through the suppression of interleukin-8 secretionThyagarajan et al. [116]33Antitumor activity of extracts of Ganoderma lucidum and their protective effects on damaged HL-7702 cells induced by radiotherapy and chemotherapyWang and Weng[117]34Reishi polysaccharides induce immunoglobulin production through the TLR4/TLR2-mediated induction of transcription factor Blimp-1Lin et al. [118]35Polysaccharide purified from Ganoderma lucidum induces gene expression changes in human dendritic cells and promotes T helper 1 immune response in BALB/c miceYu et al. [119]36Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells.Xie et al. [120]37Ganoderma lucidum extract stimulates glucose uptake in L6 rat skeletal muscle cellsJung et al. [121]38Effects of water-soluble Ganoderma lucidum polysaccharides on the immune functions of patients with advanced lung cancerGao et al. [122]
Table 3. Significant biological applications of Ganoderma lucidum polysaccharides
Conclusion
The Ganoderma lucidum mushroom is consumed commercially all over the world, because of its unique taste and curative properties. Because of the presence of numerous bioactive compounds, this mushroom is a popular herb as it contains a good amount of polysaccharides, which can be extracted by the ethanol-water solution. These polysaccharide molecules when absorbed into the human blood circulatory system, stimulates the immune modulators by activating the cellular and humoral components and increased production of macrophages. Based on the particle size and extraction time, the most common approach of hot water extraction (HWE) and ultrasound microwaveassisted extraction (UMAE), of water-soluble polysaccharides, is feasible economically. Also, the alkaline extraction of polysaccharides (AEP) of waterinsoluble polysaccharides resulted in good extraction yield as the alkaline treatment easily breaks down the dietary fibre of Reishi mushroom and speed up the release of polysaccharide extraction. The obtained polysaccharide extract is further purified by gel filtration and affinity chromatography technique that can be useful in scientific studies.
The fractionation of polysaccharides using new methodologies utilizing conformational properties was discussed that will open avenues for functional foods and herbal drugs. The biological preclinical studies showcasing the multiple health potentials of Ganoderma lucidum polysaccharides as antitumor, anti-inflammatory, antiviral, anticancer activities etc. were reviewed for future directions.
References
- Sliva D. (2006). Ganoderma lucidum in cancer research. Leukemia. 30:767–768.
- Stanley G, Harvey K, Slivova V, et al. (2005). Ganoderma lucidum suppresses angiogenesis through the inhibition of secretion of VEGF and TGF-b1 from prostate cancer cells. Biochem Biophys. 330:46–52.
- Stamets P, Yao CDW. (2002). An Informational Treatise on Mushrooms, MycoMedia Productions, Olympia. Mycomedicinals. 46.
- Chang ST, Buswell JA. (1999). Ganoderma lucidum (Curt.: Fr.) P. Karst. Aphyllophoromycetideae — a mushrooming medicinal mushroom. Int J Med Mushrooms. 1:139–146.
- Jong S.C, Birmingham JM. (1992). Medicinal benefits of the mushroom Ganoderma. Adv. Appl. Microbiol. 37:101-134.
- Hobbs Ch. (1995). An Exploration of Tradition, Healing, and Culture. Medicinal Mushrooms. 252.
- Gao Y, Zhou Sh, Chen G, et al. (2002). A Phase I/II Study of a Ganoderma lucidum (Curt.: Fr.) P. Karst. (Ling Zhi, Reishi Mushroom) Extract in Patients with Chronic Hepatitis ÃÂÃâ. Int J Med Mushrooms. 4:50.
- Wasser SP, Weis AL. (1999). Medicinal properties of substances occurring in higher Basidiomycetes mushrooms: current perspectives. Int J Med Mushrooms. 1:31-62.
- Wasser SP. (2002). Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol Biotechnol. 60:258–274.
- Smith J, Rowan N, Sullivan R. (2002). Their Therapeutic Properties and Current Medical Usage with Special Emphasis on Cancer Treatment. Medicinal Mushrooms. 256.
- McKenna DJ, Jones K, Hughes K. (2002). The Desk Reference for Major Herbal Supplements. Reishi Botanical Medicines. 2:825-855.
- Gao Y, Lan J, Dai X, et al. (2004). A phase I/II study of Ling Zhi mushroom Ganodermalucidum. (W. Curt.: Fr.) Lloyd (Aphyllophoromycetideae) extract in patients with type II diabetes mellitus. Int J Med Mushrooms. 6:33-39.
- Mizuno T, Wang G, Zhang J, et al. (1995c). Ganodermalucidum and Ganoderma tsugae: bioactive substances and medicinal effects. Food Rev Int. 11:151-166.
- Liu GT. (1999). Recent advances in research of pharmacology and clinical applications of Ganoderma P. Karst. species (Aphyllophoromycetideae) in China. Int J Med Mushrooms. 1:63-68.
- Gao Y, Zhou Sh, Huang M, et al. (2003). Antibacterial and Antiviral Value of the Genus Ganoderma P. Karst. Species (Aphyllophoromycetideae): A Review. Int J Med Mushrooms. 5:235–246.
- BaoXF, Wang XS, Dong Q, et al. (2002). Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry. 59:175–181.
- Berovic M, Habijanic J, Zore I, et al. (2003). Submerged cultivation of Ganoderma lucidum biomass and immunostimulatory effects of fungal polysaccharides. J Biotechnol. 103:77–86.
- Chen HS, Tsai YF, Lin S, et al. (2004). Studies on the immuno-modulating and anti-tumor activities of Ganoderma lucidum (Reishi) polysaccharides Bioorg. Med Chem. 12:5595–5601.
- Kim YS, Eo SK, Oh KW, et al. (2000). Antiherpetic activities of acidic protein-bound polysacchride isolated from Ganoderma lucidum alone and in combinations with interferons. J Ethnopharmacol. 72:451–458.
- Saltarelli R, Ceccaroli P, Iotti M, et al. (2009). Biochemical characterisation and antioxidant activity of mycelium of Ganoderma lucidum from Central Italy. Food Chem. 116:143–151.
- Cheong J, Jung W, Park W. (1999). Characterization of an alkali-extracted peptidoglycan from Korean Ganoderma lucidum. Arch Pharmacal Res. 22:515-519.
- Chen JH, Zhou JP, Zhang LN, et al. (1998). Chemical structure of the water-insoluble polysaccharide isolated from the fruiting body of Ganoderma lucidum. Polym J. 30:838-842.
- Liyan Z, Yanhong D, Guitang C, et al. (2010). Extraction, purification, characterization, and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr Polymers. 80:783-789.
- Chaiyavat C, Chakkrapong K, Sasithorn S. (2010). Breaking the spores of Ganodermalucidum by fermentation with Lactobacillus plantarum. Afr J Biotechnol. 9:7379-7382.
- Chang YW, Lu TJ. (2004). Molecular characterization of polysaccharides in hot-water extracts of Ganoderma lucidum fruiting bodies. J Food Drug Anal. 12:59-67.
- Qian Y, Si WW, Yan HX, et al. (2010). HPLC analysis of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes activity and Bax, Bcl-2 expression. Int J Biol Macromol. 46:167–172.
- Pang X, Chen Z, Gao X, et al. (2007). Potential of a Novel Polysaccharide Preparation (GLPP) from Anhui-Grown Ganoderma lucidum in Tumor Treatment and Immuno stimulation. J Food Sci. 72:435-S442.
- Yu-Jie F, Wei L, Yuan-Gang Z, et al. (2009). Breaking the spores of the fungus Ganoderma lucidum by supercritical CO2. Food Chem. 112:71–76.
- Chaiyavat C, Chakkrapong K, Sasithorn S. (2010). Breaking the spores of Ganoderma lucidum by fermentation with Lactobacillus plantarum. Afr J Biotechnol. 9:7379-7382.
- Sheng QH, Zheng XN. (2010). Extraction of polysaccharide from Ganoderma lucidum and its immune enhancement activity. Int J Biol Macromol. 47:336-341.
- Liyan Z, Yanhong D, Guitang C, et al. (2010). Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr Polym. 80:783–789.
- Sheng QH, Jin Li W, Zhou W, et al. (2010). Optimization of Alkaline Extraction of Polysaccharides from Ganoderma lucidum and Their Effect on Immune Function in Mice.Molecules.15:3694-3708.
- Jinghua C, Jinping Z, Lina Z, et al. (1998). Chemical structure of the water-inoluble Polysaccharides isolated from the fruiting body of Ganoderma lucidum. Polym J.30:838-842.
- GaoQL, Yan Z, Xiao LW, et al. (2011). Response surface methodology for optimization of polysaccharides extraction by mild alkaline hydrolysis from fruiting body of medicinal mushroom, Ganoderma lucidum. J Med Plants Res.5:2064-2070.
- Sone Y, Okuda R, Wada N, et al. (1985). Structure and antitumor activities of polysaccharides isolated from fruiting body and the growing culture of mycelium of Ganoderma lucidum. Agric Biol Chem. 49:2641-2653.
- Wang G, Zhang J, Mizuno T, et al. (1993). Antitumor active polysaccharides from the Chinese mushroom Songshan lingzhi, the fruiting body of Ganoderma tsugae. Biosci Biotechnol Biochem. 57:894-900.
- Mizuno T. (1999). The extraction and development of antitumor active polysaccharides from medicinal mushrooms in Japan. Int J Med Mushrooms. 1:9-29.
- Mathlouthi M, Koenig JL. (1986) Vibrational Spectra of Carbohydrates. Adv. Carbohyd. Chem. Bi. 44:7–89.
- Zhu F, Isaacs NW, Hecht L, et al. (2006). Raman optical activity of proteins, carbohydrates and glycoproteins.Chirality.18:103-115.
- Zhu F, Isaacs NW, Hecht L, et al. (2006). Raman optical activity of proteins, carbohydrates and glycoproteins. 10th International Conference on Circular Dichroism, Destin, Florida.
- Bubb WA. (2003). NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts Magn Reso. 19A:1–19.
- Leeuwen SS, Leeflang BR, GerwigGJ, et al. (2008). Development of a 1H NMR structural-reporter-group concept for the primary structural characterisation of α-D-glucans. Carbohydr Res. 343:1114–1119.
- Xue Q. (1993). Spectroscopy in Polymer Characterization. High Education Press: Beijing, 1993:263–277.
- McbriertyVJ, Packer KJ. (1993). Nuclear Magnetic Resonance in Solid Polymers, Cambridge University Press: New York, 1993: 37–103.
- Spevacek J, Brus J. (2008). Solid-state NMR studies of polysaccharide systems. Macromol Symp. 265: 69-76.
- Pizzoferrato L Manzi P, Bertocchi F, et al. (2000). Solid-state (13) C CP MAS NMR spectroscopy of mushrooms gives directly the ratio between proteins and polysaccharides. J Agric Food Chem. 48:5484–5488.
- Urai M, Yoshizaki H, Anzai H, et al. (2007). Structural analysis of mucoidan, an acidic extracellular polysaccharide produced by a pristane-assimilating marine bacterium, Rhodococcus erythropolis PR4. Carbohydr Res. 342:933–942.
- Ciucanu I, Kerek FA. (1984.) Simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. 131:209–217.
- Abdel-Akher M, Hamilton JK, Montgomery R, et al. (1952). A New procedure for the determination of the fine structure of polysaccharide. J Amer Chem Soc. 74:4970–4971.
- Datta AK, Basu S, Roy N. (1999.) Chemical and immunochemical studies of the O-antigen from enteropathogenic Escherichia coli O158 lipopolysaccharide. Carbohydr Res. 322:219–227.
- Goh KKT, Haisman DR, Singh H.(2006). Characterisation of a high acyl gellan polysaccharide using light scattering and rheological techniques. Food Hydro colloid. 20:176–183.
- Goh KKT, Hemar Y, Singh H. (2005).Viscometric and static light scattering studies on an exopolysaccharide produced by Lactobacillus delbrueckii subspecies bulgaricus NCFB 2483. Biopolymers.77:98-106.
- Goh KKT, Pinder DN, Hall CE, et al. (2006). Rheological and Light Scattering Properties of Flaxseed Polysaccharide Aqueous Solutions. Biomacromol.7:3098-3103.
- Abu-lail NI, Camesano TA. (2003).Polysaccharide properties probed with atomic force microscopy. J Microsc. 212:217-238.
- Giannotti MI, Rinaudo M, Vancso GJ. (2007). Force Spectroscopy of Hyaluronan by Atomic Force Microscopy: From Hydrogen-Bonded Networks toward Single-Chain Behavior. Biomacromol.8:2648-2652.
- Liu C, Wang Z, Zhang X. (2006). Force Spectroscopy of Single-Chain Polysaccharides: Force-Induced Conformational Transition of Amylose Disappears under Environment of Micelle Solution. Macromol.39:3480-3483.
- Zhang Q, Marszalek PE. (2006).Identification of Sugar Isomers by Single-Molecule Force Spectroscopy. J Am Chem Soc.128:5596-5597.
- Zhang Q, Marszalek PE. (2006).Solvent effects on the elasticity of polysaccharide molecules in disordered and ordered states by single-molecule force spectroscopy. Polymer.47:2526–2532.
- Zhang L, Wang C, Wang Z, et al. (2003).Single-Molecule Force Spectroscopy on Curdlan: Unwinding Helical Structures and Random Coils. Nano Lett. 3:1119-1124.
- Meunier F, Wilkinson KJ. (2002). Nonperturbing Fluorescent Labeling of Polysaccharides. Biomacromol. 3:857-864.
- Friedman M. (2016). Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans.Foods.5:80
- Wasser SP, Weis AL. (1997). Medicinal mushrooms: Ganoderma Lucidum (Curtis: Fr.) P. Karst, Reishi mushroom.Pedelfus Pub.39.
- Gao Y, Zhou Sh, Chen G, et al. (2002). A Phase I/II Study of a Ganodermalucidum (Curt.: Fr.) P. Karst. (Ling Zhi, Reishi Mushroom) Extract in Patients with Chronic Hepatitis ÃÂÃâ. Int J Med Mushrooms .4:50.
- Gao Y, Zhou Sh, Huang M, et al. (2003).Antibacterial and Antiviral Value of the Genus Ganoderma P. Karst. Species (Aphyllophoromycetideae): A Review. In. J Med Mushrooms.5:235–246.
- Gao Y, Lan J, Dai X, et al. (2004).A phase I/II study of Ling Zhi mushroom Ganodermalucidum. (W. Curt.: Fr.) Lloyd (Aphyllophoromycetideae) extract in patients with type II diabetes mellitus. Int J Med Mushrooms.6:33-39.
- Chen AW, Miles PG. (1996). Biomedical research and the application of mushroom nutraceuticals from Ganodermalucidum. In Mushroom Biology and Mushroom Products, Royse DJ Ed. 161-175.
- Sone Y, Okuda R, Wada N. (1985). Structure and antitumor activities of polysaccharides isolated from fruiting body and the growing culture of mycelium of Ganodermalucidum. Agric Biol Chem.49:2641-2653.
- Wang SY, Hsu ML, Hsu HC. (1997).The anti-tumor effect of Ganodermalucidumis mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer.70:699-705.
- Hsu MJ, Lee SS, Lin WW. (2002).Polysaccharide purified from Ganodermaluciduminhibits spontaneous and Fas-mediated apoptosis in human neutrophils through activation of the phosphatidylinositol-3 kinase/Akt pathways. J Leuokoc Biol.72:207-216.
- Hsu MJ, Lee SS, Lee ST. (2003).Signalling mechanisms of enhanced neutrophil phagocytosis and chemo taxis by the polysaccharide purified from Ganodermalucidum. Brit J Pharmacol.139:289-298.
- Kim HS, Kacew S, Lee BM. (1999).In vitro chemopreventive effects of plant polysaccharides (Aloe barbadendis Miller, Lentisedodes, Ganodermalucidum and Coriolus versicolor).Carcinogenesis. 20:1637-1640.
- Lee JM, Kwon H, Jeong H, et al. (2001).Inhibition of lipid peroxidation and oxidative damage by Ganodermalucidum. Phytother Res.15:245-249.
- Wang YY, Khoo KH, Chen ST, et al. (2002).Studies on the immuno-modulating and antitumor activities of Ganodermalucidum(Reishi) polysaccharides: functional and proteomic analysis of a fucose-containing glycoprotein fraction responsible for the activities. Bioorgan Med Chem.10:1057-1062.
- Zhang J, Tang Q, Zimmerman-Kordman M, et al. (2002).Activation of B lymphocytes by GLIS, a bioactive proteoglycan from Ganodermalucidum. Life Sci. 71:623-638.
- Mizushina Y, Hanashima T, Yamaguchi L, et al . (1998).A mushroom fruiting body-inducing substance inhibits activities of repli-cative DNA polymerases. Biochem.Biophys.Res. Commun.249:12-22.
- Shiao MS, Lee KR, Lin LJ, et al. (1994).Natural products and biological activities of the Chinese medicinal fungus, Ganodermalucidum. Am Chem Soc.26:342-354.
- Ourission G. (1983).Les acidesganoderiques T & Z: triterpenes cytotoxiquesdeGanodermalucidum (Polyporacee). Tetrahedron Lett. 24:1081-1084.
- Min BS, Nakamura N, Miyashiro H, et al. (1998).Triterpenes from the spores of Ganodermalucidum and their activity against HIV-1protease. Chem Pharm Bull. 46:1607-1612.
- El-Mekkawy S, Meselhy MR, Nakamura N, et al. (1998). Anti-HIV-1 and antiHIV-1-protease substances from Ganodermalucidum. Phytochemistry. 49:1651-1657.
- Min BS, Gao JJ, Nakamura N, et al. (2000).Triterpenes from the spores of Ganodermalucidumand their cytotoxicity against Meth-A and LLC tumor cells. Chem Pharm Bull.48:1026-1033.
- Kimura Y, Baba M, Taniguchi K. (2002).Antitumor and antimetasticeffects on liver triterpenoid fractions of Ganodermalucidum: mechanism of action and isolation of an active substance. Anticancer Res. 22:3309-3318.
- Mau JL, Lin HC, Chen CC. (2002). Antioxidant properties of several medicinal mushrooms. J Agric Food Chem. 50:6072-6077.
- Liu X, Yuan JP, Chung CK, et al. (2002). Antitumor activity of the sporoderm-broken germinating spores of Ganoderma lucidum. Cancer Lett.182:155-161.
- Lawal TO, Wicks SM, Mahady GB, et al. (2017). The Impact of Chemistry on Biological Activity in Cancer. Current Bio Comp. 13:2017.
- Zeng P, Guo Z, Zeng X, et al. (2018). Chemical, biochemical, preclinical and clinical studies of Ganoderma lucidum polysaccharide as an approved drug for treating myopathy and other diseases in China. J Cell Mol Med. 22:3278–3297.
- Zengtao X, Xiuping C, Zhangfeng Z, et al. (2011). Ganoderma lucidum Polysaccharides: Immunomodulation and Potential Anti-Tumor Activities. AM J Chinese Med. 39:15-27.
- Shang D, Li Y, Wang C, et al. (2010). A novel polysaccharide from Se-enriched Ganoderma lucidum induces apoptosis of human breast cancer cells. Oncol Rep. 25:267-272.
- Chen WY, Yang WB, Wong CH, et al. (2010). Effect of Reishi polysaccharides on human stem/progenitor cells. Bioorg Med Chem. 18:8583–8591
- Lin CY, Chen YH, Lin CY, et al. (2010). Ganoderma lucidum Polysaccharides Attenuate Endotoxin-Induced Intercellular Cell Adhesion Molecule-1 Expression in Cultured Smooth Muscle Cells and in the Neointima in Mice. J Agric Food Chem. 58:9563-9571.
- Huang CY, Chen JY, Wu JE, et al. (2010). Ling-Zhi polysaccharides potentiate cytotoxic effects of anticancer drugs against drug-resistant urothelial carcinoma cells. J Agric Food Chem. 58:8798–8805.
- Lai CY, Hung JT, Lin HH, et al. (2010). Immuno-modulatory and adjuvant activities of a polysaccharide extract of Ganodermalucidum in vivo and in vitro. Vaccine. 28:4945-4954.
- Chia JW, Gow CY. (2010). The in vitro and in vivo experimental evidences disclose the chemopreventive effects of Ganoderma lucidum on cancer invasion and metastasis. Clin Exp Metastasis. 27:361-369.
- Eva C, Jose LM, Pilar S, et al. (2010). Ganodermalucidum induced apoptosis in NB4 human leukemia cells: involvement of Akt and Erk. J Ethnopharmacol. 128:71-78.
- Jie L, Kuniyoshi S, Ryuichiro K. (2010).The effects of ganoderma alcohols isolated from Ganoderma lucidum on the androgen receptor binding and the growth of LNCaP cells. Fitoterapia. 81:1067-1072.
- Nian HC, Jian WL, Jian JZ. (2010). Ganoderic acid T inhibits tumor invasion in vitro and in vivo through inhibition of MMP expression. Pharmacol Rep. 62:150-163.
- Sudheesh NP, Ajith TA, JanardhananKK. (2009). Ganoderma lucidum (Fr.) P.Karst enhances activities of heart mitochondrial enzymes and respiratory chain complexes in the aged rat. Biogerontology. 10:627-636.
- Lin YL, Liang YC, Tseng YS, et al. (2009). An immunomodulatory protein, Ling Zhi-8, induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and MAPK pathways. J Leukocyte Biol. 86:877-889.
- Weng CJ, Chau CF, Yen GC, et al. (2009). Inhibitory effects of Ganoderma lucidum on tumorigenesis and metastasis of human hepatoma cells in cells and animal models. J Agric Food Chem. 57:5049-5057.
- Kazem A, Majid R. (2009). Ganoderma lucidum induces the expression of CD40/CD86 on peripheral blood monocytes. Iran J Immunol. 6:87-91.
- Ajith TA, Sudheesh NP, Roshny D, et al. (2009). Effect of Ganoderma lucidum on the activities of mitochondrial dehydrogenases and complex I and II of electron transport chain in the brain of aged rats. Exp Geronto. 44:219-23.
- Pinweha S, Wanikiat P, Sanvarinda Y, et al. (2008). The signaling cascades of Ganoderma lucidum extracts in stimulating non-amyloidogenic protein secretion in human neuroblastoma SH-SY5Y cell lines. Neurosci Lett. 448:62-66.
- Fukuzawa M, Yamaguchi R, Hide I, et al (2008). Possible involvement of long chain fatty acids in the spores of Ganoderma lucidum (ReishiHoushi) to its anti-tumor activity. Biol Pharm Bull. 31:1933-1937.
- Zhang Y, Lin Z, Hu Y, et al. (2008). Effect of Ganoderma lucidum capsules on T lymphocyte subsets in football players on "living high-training low". Br J Sports Med. 42:819-822.
- Li L, Li T, Wang XJ, et al. (2008). Effects of Ganoderma lucidum spores on HepG2 cells proliferation and growth cycle. Zhong Yao Cai. 31:1514-1518.
- Noguchi M, Kakuma T, Tomiyasu K, et al. (2008). Randomized clinical trial of an ethanol extract of Ganoderma lucidum in men with lower urinary tract symptoms. Asian. J Androl. 10:777-785.
- Ying BL, Rui W, Hong LW, et al. (2008). Serum amyloid A mediates the inhibitory effect of Ganoderma lucidum polysaccharides on tumor cell adhesion to endothelial cells. Oncol Rep.20:549-556.
- Yuen JWM, Gohel MDI. (2008). The dual roles of Ganoderma antioxidants on urothelial cell DNA under carcinogenic attack. J Ethno pharmacol. 8:324-330.
- Wing KC, Christopher CHC, Helen KWL, et al. (2008). Ganoderma lucidum polysaccharides can induce human monocyticleukemia cells into dendritic cells with immuno-stimulatory function. J Hematol Oncol. 1:1-12
- Noguchi M, Kakuma T, Tomiyasu K, et al. (2008). Effect of an extract of Ganoderma lucidum in men with lower urinary tract symptoms: a double-blind, placebo-controlled randomized and dose-ranging study. Asian J Androl.10:651-658.
- Yuen JWM, Gohel MDI, Au DWT. (2008).Telomerase-associated apoptotic events by mushroom Ganoderma lucidum on premalignant human urothelial cells. Nutr Cancer. 60:109-119.
- Kun CC, Hsuan CH, Jenn HC, et al. (2007). Ganoderma lucidum polysaccharides in human monocyticleukemia cells: from gene expression to network construction. BMC Genomics. 8:1-17.
- Hijikata Y, Yamada S, Yasuhara A. (2007). Herbal mixtures containing the mushroom Ganoderma lucidum improve recovery time in patients with herpes genitalis and labialis. J Altern Complement Med. 13:985-987.
- ZaidmanBZ, Wasser SP, Nevo E, et al. (2007). Androgen receptor-dependent and -independent mechanisms mediate Ganoderma lucidum activities in LNCaP prostate cancer cells. Int J Oncol. 31:959-967.
- Hua KF, Hsu HY, Chao LK, et al. (2007). Ganoderma lucidum polysaccharides enhance CD14 endocytosis of LPS and promote TLR4 signal transduction of cytokine expression. J Cell Physiol. 212:537-550.
- Pang X, Chen Z, Gao X, et al. (2007). Potential of a Novel Polysaccharide Preparation (GLPP) from Anhui-Grown Ganoderma lucidum in Tumor Treatment and Immunostimulation. J Food Sci. 72:S435-S442.
- HoYW, Yeung JS, Chiu PK, et al. (2007). Ganoderma lucidum polysaccharide peptide reduced the production of proinflammatory cytokines in activated rheumatoid synovial fibroblast. Mol Cell Biochem. 301:173-181.
- Thyagarajan A, Jiang J, Hopf A, et al. (2006). Inhibition of oxidative stress-induced invasiveness of cancer cells by Ganoderma lucidum is mediated through the suppression of interleukin-8 secretion. Int J Mol Med. 18:657-664.
- Wang DH, Weng XC. (2006). Antitumor activity of extracts of Ganoderma lucidum and their protective effects on damaged HL-7702 cells induced by radiotherapy and chemotherapy. Yao Za Zhi. 31:1618-1622.
- Lin KI, Kao YY, Kuo HK, et al. (2006). Reishi polysaccharides induce immunoglobulin production through the TLR4/TLR2-mediated induction of transcription factor Blimp-1. J Biol Chem. 281:24111-24123.
- Yu LL, Shiuh SL, Shin MH, et al. (2006). Polysaccharide purified from Ganoderma lucidum induces gene expression changes in human dendritic cells and promotes T helper 1 immune response in BALB/c mice. Mol Pharmacol. 70:637-644.
- Xie JT, Wang CZ, Wicks S, et al. (2006). Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Exp Oncol. 28:25-29.
- Jung KH, Ha E, Kim MJ, et al. (2006). Ganoderma lucidum extract stimulates glucose uptake in L6 rat skeletal muscle cells. Acta Biochim Pol. 53:597-601.
- Gao Y, Tang W, Dai X, et al. (2005). Effects of water-soluble Ganoderma lucidum polysaccharides on the immune functions of patients with advanced lung cancer. J Med Food. 8:159-168.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
