Emerging Trends, Challenges and Prospects in Antiviral Therapeutics and Drug Development for Infectious Diseases
Shailendra K. Saxena, Shailja Saxena, Rakhi Saxena, ML Arvinda Swamy, Ankur Gupta, Madhavan P.N. Nair
Shailendra K. Saxena1,*,Shailja Saxena2,Rakhi Saxena1,ML Arvinda Swamy1,Ankur Gupta3,Madhavan P.N. Nair4
1Centre for Cellular and Molecular Biology,Uppal Road,Hyderabad 500 007 (AP),India
2CSM Medical University (King George’s Medical College),Lucknow 226 003 (UP),India
3J.S.S. College of Pharmacy,Ootacamund 643 001 (TN),India
4College of Medicine,Florida International University,Miami 33199 (FL),USA.
- Corresponding Author:
- Shailendra K. Saxena
Tel: +91-40-27192630
Fax: +91-40-27160591
E-mail: shailen@ccmb.res.in
Abstract
Infectious diseases are well known since ancient time to human civilization. Different microorganisms (bacteria, fungi, viruses) cause infectious diseases. Among all the microorganisms, viruses are the most notorious. Viruses are ultra microscopic obligate intracellular parasites, which have either DNA or RNA as genetic material, known to cause different types of diseases in humans, animal and plants. The battle between the viruses and the humans is continuous process, as both will adopt different strategies to combat against each other. Development of antiviral drugs is very tedious process as it involves many stages like, target identification and screening, lead generation and optimization, pre-clinical and clinical studies, final registration of the drug etc. Inspite of modern tools and stringent quality control measures only a few antiviral drugs are getting approved for human use. The reason may be either side effects or the antiviral drug resistance. Increasing knowledge about viruses, mechanism of their infections and the rapid evolvement of novel antiviral strategies and techniques will speed up the development of novel antiviral drugs. This review is focused on global view of drug discovery for infectious diseases including strategies, current advanced techniques and tools, major landmarks and limitations of the antiviral drug development in last five decades.
Keywords
Virus; antiviral drug; infectious diseases; clinical trials; therapeutics.
1. Introduction
Infectious diseases are known since more than 1000 B.C. [1,2]. Protection and remedies against these infectious diseases always remain prime concern for the human Infectious diseases are a leading cause of death in the world. Of the 54 million deaths that occurred in 1998,the infectious diseases alone accounted for one fourth to one third [3]. It has been estimated that in South East Asian region,they are responsible for about 40% of the 14 million deaths occurring annually in the region and account for 28% of the global burden of infectious diseases. [4].
Among all the organisms causing diseases in animals,viruses are the most notorious. There are about 87 commonly known viral diseases affecting animals. Viruses are obligate parasites having DNA or RNA as their genetic material enclosed within a coat called capsid. These viruses are the most active and important members of the disease causing community of the microbes,blessed with the remarkable gift of being able to evolve rapidly. Unlike bacteria,which are relatively large organisms and have their own unique metabolism,viruses are small and utilize a lot of host factors for replication. Antiviral drugs can be toxic to human cells. The number of viruses that have newly emerged in the last 30 years is more than 30,and these are the ones,responsible for the major disease outbreaks [4].
Drugs that are used to treat viral infections are called antiviral drugs. The mechanism of action of the antiviral drugs can vary,ranging from targeting viral proteins to cellular proteins and strengthening the immune response to the viral infection. The drugs that aim at strengthening the immune response include several types of interferons,immunoglobulins,and vaccines. Interferon drugs are like the naturally occurring substances that slow or stop viral replication whereas Immune globulin is a sterilized solution of antibodies collected from a group of people and vaccines help prevent infection by stimulating the body's natural defense mechanisms. Generally immune globulins and vaccines are given before a person is exposed to a virus in order to prevent infection. However some immune globulins and vaccines,(for rabies and hepatitis B),are also used after exposure to the virus to prevent the development of the infection in to a more severe form.
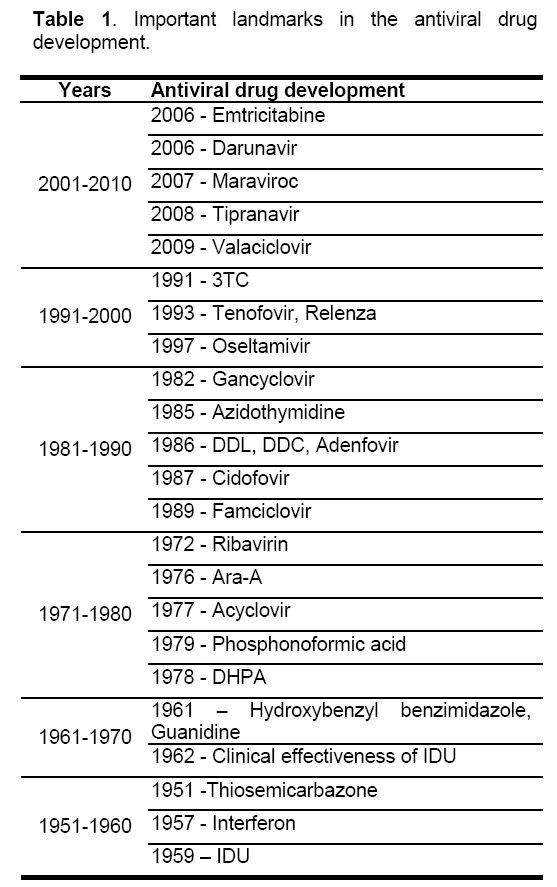
The contributions from various researchers from cell biology,genetics,and molecular biology helped in understanding the structure and molecular functions of the virus. the knowledge gained from the combined efforts of this research helped in developing new antiviral. The antiviral drugs can be given orally,intravenously,intramuscularly,in the form of ointments,creams,or eye drops or are inhaled as a powder. The first successful administration of antiviral drugs to patients began in 1960’s,the drug being thiosemicarbazone to treat small pox by Bauer [5]. The important milestones in the antiviral drug development are given in the (Table 1). Till now nearly 42 antiviral drugs have been developed and many more are in clinical trials [6,7,8,9,10,11,12,13,14]. However,Viruses can develop resistance to antiviral drugs. So,developing antiviral drugs is a difficult task.
2. Classification of Chemotherapeutic Agents Againest Viruses
Basically the chemotherapeutic agents against viral infections can be categorised in to following the three main categories.
2.1 Virucides
These are the agents or drugs that inactivate intact viruses. They cause inactivation of the virus in a single step. These affect both the viral cell and the host cell,so are not used generally. However they can be used in preventing the transmission of viruses. The examples include organic solvents,detergents and ultravoilet light.
2.2 Antivirals
These are the agents or drugs that inhibit viral replication at certain levels. These specifically have a restricted spectrum of activity and cannot be used for latent viruses.
2.3 Immunomodulators
These consist of agents that augment the host response to infections. They help in boosting up the host immune response by secreting antibodies,interferons or by intensifying the cell mediated immunity. Antiviral drugs have a very important role in preventing the spread of viral diseases. An antiviral drug must have the following important charecteristics:
It must be able to reach the target organ which the virus is enhabiting.
It must be chemically and metabolically stable.
It must specifically inhibit virus function without affecting the host functions.
It should be readily absorbed
It should not be toxic,carcinogenic,allergenic or mutagenic.
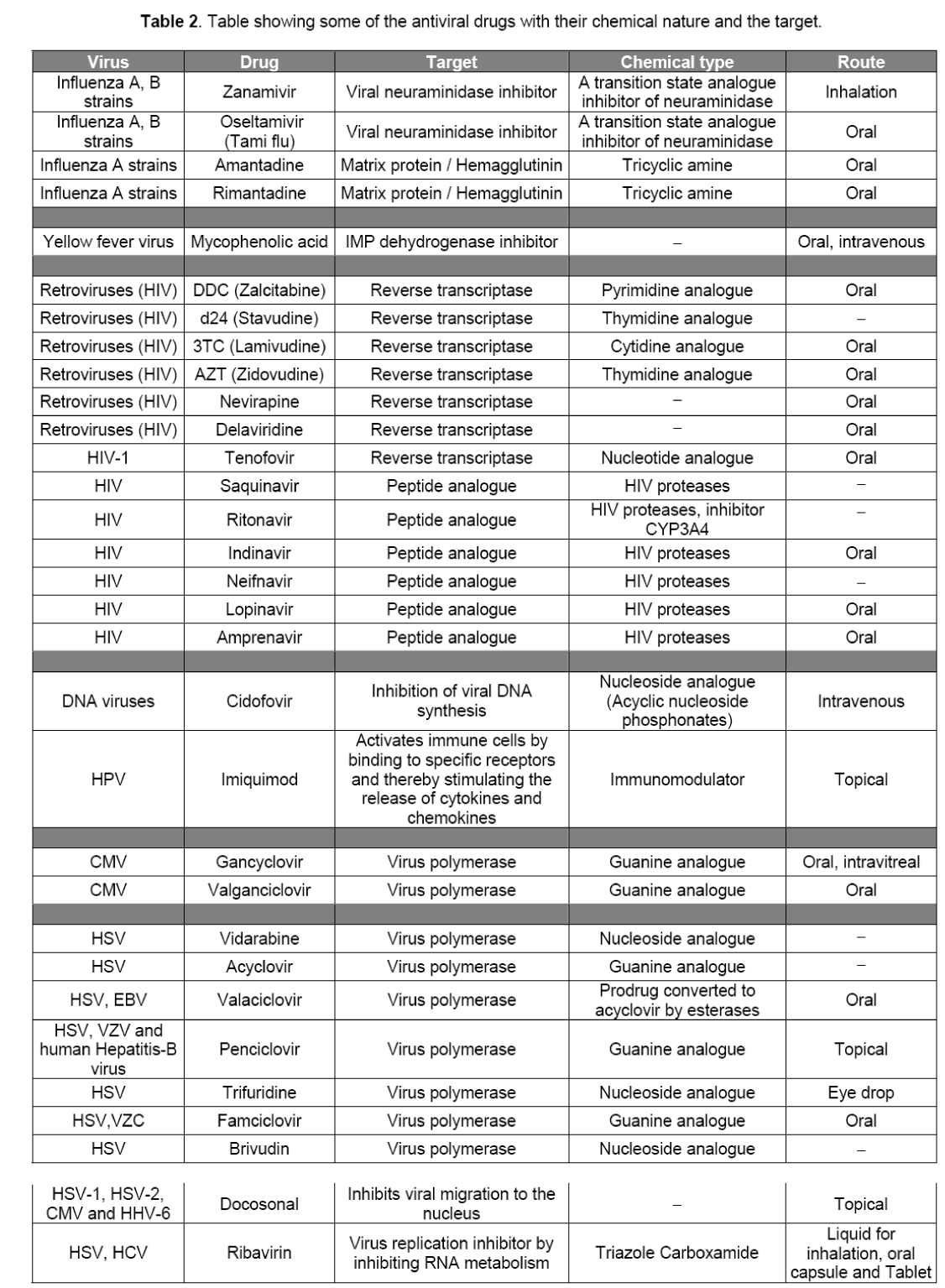
The third point is very critical because some viruses utilize host cell machinery to replicate. And it was because of this fact that initially it was very difficult to specifically block viral replication inside the host cells. The development of molecular biology and virology helped us to understand the intricacy of viral multiplication,unfolding a lot of important features of these notorious creatures that helped us to target specifically virus functions inside the cell for inactivation. Because of this,many antiviral drugs and highly effective vaccines have been developed to treat the viral diseases. The first antiviral drug dates back to 1960,which was developed to deal with herpes virus,causing some common infections in humans like chicken pox,measles,and Kaposi’s sarcoma etc. It was developed mainly on the basis of trial and error method,so the process of its development was very time consuming. The drug also showed a few side effects. With the full genetic sequences of viruses being worked out till 1980s,researchers came to know about the exact mechanism of working of viruses and the drugs that can be specifically and actively be directed against them. As a result many antiviral drugs have been now developed and many more are in the process of development. Some of them are given in Table 2. Drug Development is a highly demanding job. Lot of challenges will be faced by pharmaceutical industries in drug discovery as it involves huge money and time for R&D,many phase trials,and finally approval of the drugs to market) (Nearly it takes 10 to 15 years and 1 billion dollars to bring a new drug into the market much of this expenses can be attributed to the costly late stage failures Nearly 10–15 years and almost USD 1 billion are spent to bring a new drug to market. Much of this expense can be attributed to the costly late-stage failures [15]. By 2010,the cost of successfully developing a new drug is expected to reach USD 2 billion if the efficiency and effectiveness of the drug development process are not improved [16].
3. Development of Antiviral Drug
The drug development process traditionally involves utilizing in vivo screens,which is a time consuming process. It involves a lot of study regarding the potential drug candidate including its pharmacokinetic properties,metabolism,and toxicity. The drug development process involves some basic steps [17] given below:
Target identification and screening
Lead generation and optimization
Pre-clinical and clinical studies
Final registration of the drug
3.1 Target identification and screening
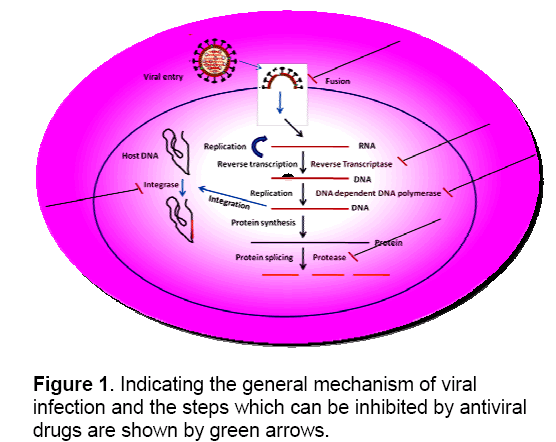
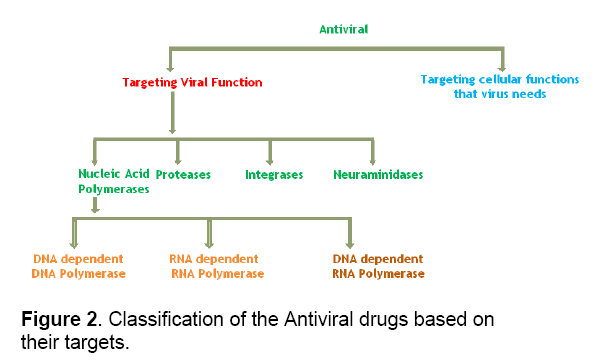
The potential targets,which can be used for the development of antiviral drugs,are either viral genome or the host genome. In Most of the cases the virus genome is targeted because the viral genome size being small,very few number of potential targets and moreover it is less toxic to the host. So it is important to identify the cellular components necessary for viral propagation. There are many steps in the viral life cycle that can be targeted (Figure 1). On the basis of the nature of the target the antiviral drugs can be classified (Figure 2 ).
First of all the mechanism of action of the antiviral drug is to be studied. The ability of the drug to specifically inhibit viral replication should be demonstrated by biochemical,cellular and structural data and then the site of action of the drug should be established. It is especially important to test the specificity of the drug for the viral target over cellular proteins in cases where viral enzyme has a cellular counterpart.
The second step includes testing the effect of the antiviral drug in vitro. The drug should show specific quantifiable antiviral activity in vitro. The in vitro antiviral activity and cytotoxicity assessment should also be done in order to select for appropriate dose ranges in early clinical trials. Antiviral activity evaluations that are recommended by FDA to support the development of the investigational drug include:
Assessment of specific antiviral activity of the drug against a broad range of clinical and laboratory viral isolates.
Evaluation of the antiviral activity of the drug against mutant viruses that are resistant to drugs with the same molecular target as the drug under study as well as viruses resistant to other drugs for the same indication.
The antiviral activity can be tested by a number of assays namely virus inactivation assays,plaque reduction assays,cytopathic effect inhibition assays,peripheral blood mononuclear cell (PBMC) assays,and binding and fusion assays. Determination of the IC50 and intracellular half-life of the triphosphate form of the drug in dividing and non-dividing cell in case of nucleoside analogues should be determined. In the absence of animal and cell culture models for the human viruses,inhibition of viral activity or viral function of drug can be measure of its activity. One more important factor to consider is the interference of serum protein to the action of the drug under study.
The antiviral activity of the drug can also be assessed by the measurement of the viral titer in animal model systems after drug treatment. Cytotoxicity tests should also be done in order to find whether the drug is safe to the host cells or not. This value is referred to as the median cellular cytotoxicity concentration and is identified by CC50 or CCIC50. It is desirable to have a high therapeutic index (given by CC50 value/IC50) giving maximum antiviral activity with minimal cell toxicity. The therapeutic index should be calculated before phase I trials. The effect of the drug on mitochondrial toxicity should also be examined.
3.2 Lead generation and optimization
This is one of the most important and the ratedetermining step in the drug discovery process. The lead generation can be done by a number of strategies that include substrate based (for enzymes),screening,and biostructural approaches. The substrate-based approach has been applied to targets such as HIV proteinase inhibitors,Influenza virus neuraminidase inhibitors. The lead compounds can also be obtained by screening libraries of compounds [18].
3.3 Pre-clinical and clinical studies
The pre clinical studies are done to study of the toxicity of drugs,the affect of drugs in vitro and in vivo by performing the genotoxicity testing and investigation on drug absorption and metabolism. It also involves the study of toxicity of drug metabolites and the rate of excretion of the drug and its metabolites. According to FDA,the important facts that need to be addressed include:
Pharmacological profile of the drug.
Acute toxicity of drug in at least two species of animals.
Short term toxicity studies depending on proposed duration of use of the drug.
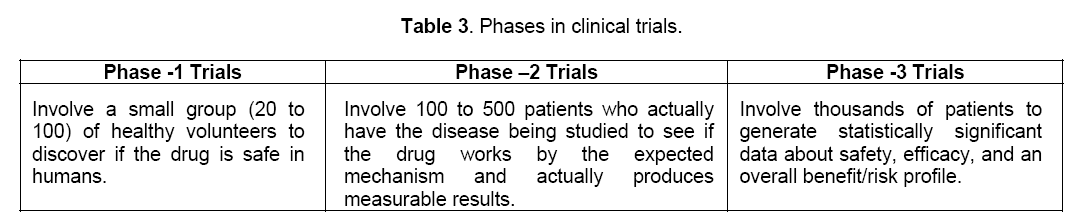
The drug or drugs,which have passed all the tests in pre-clinical tests,may be the ideal candidate for clinical trials. Before starting any clinical trial with the drug the investigators or the company should file an application (IND) investigational new drug with the U.S. Food and Drug Administration (FDA). FDA reviews the application to make sure patients involved in the clinical trials will not face unreasonable risks. Once the IND application has been approved,clinical trials (Table 3) begin [19].
3. 4 Final registration of the drug
After the drug is tested preclinically and clinically,it is made available for public use.
4. Techniques
Many new techniques have been introduced into drug discovery and development in order to speed up the process and overcome the high costs. These tools include genomics,proteomics,metabolomics,biomarkers,bioinformatics,and molecular imaging techniques.
4.1 Molecular Imaging
The molecular imaging technique can be used for various different purposes,which include:
Anatomical studies for structural morphology of organs or tissues,including computerized tomography (CT),magnetic resonance imaging (MRI),and ultrasound.
Techniques used for visualization of biological processes in tissues and organs at cellular and molecular level include optical imaging (Bioluminescence and fluorescence),Positron emission tomography (PET),and Photon emission tomography (SPECT).
The combination of structural and functional imaging capabilities that help in enabling accurate localization of biological or pathophysiological processes in tissues or organs,including PET/CT,SPECT/CT,and MRI/PET.
Biggest challenges faced in drug development are the translation of pre-clinical results to clinical trials,failures rates up to 50% depends on the lack of safety or efficacy. This gap can be minimized by using PET and SPECT in vivo imaging information on drug effects in experimental animal models and humans. It is still under development process. This reduces the late stage failures,enhances the selection of the ideal candidate and more over it is less expensive.
Radios labeled tracers are involved in nuclear imaging with PET or SPECT for visualizing the specific biological process at the cellular and molecular level. Key considerations for these are the development radiolabeled tracer designs. Therefore for the development of these radio tracer designs are key consideration for drug development. Generally three different approaches can be taken to develop radio labeled tracers for use in drug development:
Radio labeled drug can be used to access the bio-distribution and PK/ ADME characteristics.
Radio ligands can be used for the drug targets to access the PD properties of drug.
Radio labeled biomarkers can be used to access the efficacy of the drug candidates.
Isotopes used for imaging are either gamma emitters for SPECT imaging or positron emitters for PET imaging. When selecting radioisotope for nuclear imaging studies,several factors must be taken under consideration:
Most important consideration is the physical half-life of the radioisotope,which should be long to meet the requirements of the study.
The biological half-life of the molecule to be radio labeled
Radiolabeling should not be effected by the interference of physical and chemical contaminants like radiation,isotope specific activity and trace metal contamination [16].
4.2 Role of DNA microarray
DNA microarray is an important tool that has been efficiently utilized in the development of host-protein target. The technique has several advantages the major one being the analysis of a large number of samples. DNA microarray has been used to study the host response to HCMV infection [20]. The changes in cellular gene expression during viral infection may be due to cellular defense response or a virally induced response that is essential for the virus. By identifying variations in the drug metabolism genes like CYP2D6 and CYP2C19 will help in assessing how the individual metabolizes the different drugs used for treatment [21]. The analysis of the global response to infection using microarray is an important way to find new treatments in relatively few steps and thus pave the path for the development of new antiviral drugs.
4.3 Biomarkers
Biomarkers are signature molecules,which can indicate physiological or biological characteristics changes in the specific target organs. Biomarkers can be used for various purposes like diagnosis,identifying the organs involved,identifying the risk of disease,assessing weather the drug administered is safe and effective in treating the disease. For Example biomarkers used for HIV/AIDS can access viral load (the number of free virus particles in the blood) and can count the CD4 positive immune cells [17,22].
4.4 Animal Testing
Drugs are administered to the Animals for measuring the drug level in the blood,its metabolism,excretion and toxicity of its metabolites. Drug testing can be performed on animal in two ways:
1. Short term drug testing which is for a period of 2 to 3 weeks or months.
2. Long term drug testing on animals can be carried for few weeks to several years in orders to see the long term ill effects of the drug like cancers and birth defects etc.
4.5 Bioinformatics
Drug development in conventional method involves random screening of the several compounds for antiviral activity in cell culture and identifying very few compounds. For few compounds structural modifications can be incorporated to them and finally resting them for the antiviral activity. This is very time and resource consuming process) (now a days the use of computational techniques revolutionized the drug discovery by using the different computer aided software’s like CADD (computer aided drug design) CAMM (computer aided molecular modeling) CADDD (computer aided drug discovery and development) in silico drug design and rational drug designs minimized the cost and time in screening the ideal drug candidates [23]. Computer aided drug design involves determination of the three dimensional structure of the viral molecules using X-ray crystallography. This is followed by computer simulation and thermodynamic computation to study the atomic interaction with other molecules in order to screen out the structure with favorable binding characteristics [5]. Computer aided drug design is still under development and no antiviral agent has yet been designed de novo by this technique. However,the rapid advances in this field hold great promise for the efficient,selective identification of new drugs.
Using this approach,it is much easy to identify the active drug candidates (hits),select the most likely candidate that can be made to proceed for further evaluation (selection of leads) and finally transform these active biological molecules to suitable drugs by improving their physicochemical,pharmaceutical,ADMET/PK (absorption metabolism excretion toxicity/pharmacokinetic) properties. Thus the in silico model leads to the significant reduction in time and resource requirement of chemical synthesis and biological testing [23].
4.6 QSAR
Quantitative structure-activity relationship (QSAR) The fundamental principle of QSAR is that biological properties are functions of molecular structure. (QSAR is often used in the drug discovery to identify the chemical structures which show inhibitory effects on specific targets more over this process is used to for quantitative approach for biological activity or chemical activity. In one of the studies,QSAR was used to study HIV-1 integrase inhibition [24].
4.7 Tissue microarray
Tissue micro array chips are used for testing,evaluation and efficacy of the drug distribution and validating the targets. These microchips are generally coated with numerous normal and diseased human tissues for high throughput screening of the targets [25].
4.8 Fluorescence based approaches
Targeting proteases have been an important approach for combating viral infection as viruses use the cellular or their own proteases for purposes like membrane fusion or the activation of their surface proteins. This makes the protease inhibitors active drug candidates as an example,the drugs like Ritonavir,Nelfinavir,Saquinavir and Lopinavir are HIV-1 protease inhibitors,which are used to treat HIV-1 infection [26].
4.9 HPLC
Various techniques are used for the analysis of the drugs HPLC is used widely in the analysis of the drugs for example Oseltamivir [27,28].
5. Problems
Viruses have a high rate of multiplication and also a very high rate of mutation. The viral resistance is the cause of this genetic mutation,which results in either the change in some enzymes or the structural component of the virion. The high rate of virus multiplication inside the host cell results in the creation of a larger gene pool from which the mutations can appear. The selective pressure caused by the antiviral agents under these circumstances results in the proliferation and transmission of resistant viruses resulting in the replacement of susceptible population with the resistant one. The Immunocompromised patients are the most at risk. It is evident from the report showing that AIDS patients suffer with serious illness from resistant herpes viruses [29].
The next important issue to be addressed is cross-resistance among anti-viral drugs. Resistance to one drug is accompanied by the declined susceptibility to another drug of the same class. However cross-reactivity between different classes of drugs is also reported. The treatment with acyclovir to acyclovir- resistant herpes virus in AIDS patients results in failure of the healing of herpes lesions. However this is not the case with immunocompromised patients. Thus viral resistance is clinically important in some cases. However,this is still to be completely studied.
The identification of viral resistance is very important. New methods are being developed for measuring viral resistance the major ones being – Polymerase Chain Reaction (PCR) for the identification of resistant genes,the use of cell lines that allow the use of broader spectrum of viruses,and some of the improved methods of nucleic acid hybridization. In order to minimize resistance several measures have been suggested which include use of combination therapy wherever applicable,avoiding prolonged use of antiviral drugs and discontinuing the use of the drug whenever possible.
5.2 Latency
The antiviral drugs are “virustatic “instead of being virucidal. As soon as the drugs are removed the virus again starts proliferating. Thus the anti-viral drugs fail to act on latent viruses. As evident from the fact that treatment with acyclovir does not prevent future recurrences of HSV. The presence of latent virus in the host results in prolonged contact of the virus with the drug,and this may result in the production of resistance. The study of latency-associated factors in the virus may help in solving this problem.
5.3 Immunosuppression by antiviral drugs
Some of the antiviral drugs results in the suppression of the immune response of the host cells. The antiviral drugs are known to have antiproliferative activity against the rapidly multiplying cells. Some of the antiviral drugs have been shown to decrease mitogenesis. Unwanted side effects of antiviral agents should be identified in order to minimize the immunosuppressive properties of the antiviral [5].
5.4 Toxicity due to antiviral drugs
Toxicity is a major problem with all anti-virus drugs because of the drugs being not selective enough which may result in their interference with normal cellular enzymes and DNA synthesis. For example,AZT used against AIDS is so toxic that the seriously ill AIDS patients can not take it. The toxic effects of AZTinclude decrease in the total white blood count,a direct decrease in the number of T cells and hemotocrit drops in the patients. If used improperly,AZT can cause late drug toxicity with drug-induced immunosuppression. Serious side-effects of AZT is unavoidable because AZT is blocker of DNA synthesis [30].
5.5 Improper diagnosis
The major difficulty with the viral infections is early diagnosis of the infection the timely treatment of many viral infections such as infections of the respiratory tract. Proper diagnosis is very important for the disease to be treated. However in the cases where the symptoms are mild or the symptoms appear late,the disease may remain undiagnosed or sometimes misdiagnosed. This results in delay in the treatment of the disease.
6. Alternative Approaches
Apart from antiviral drugs there are some alternative approaches that are used for the treatment of viral diseases. These approaches include vaccines,immunomodulators,antisense oligonucleotides,ribozymes and aptamers.
6.1 Use of antisense oligonucleotides
The antisense nucleotide approach involves targeting of specific nucleotides rather than proteins. Fomivirsen is a phosphorothioate oligonucleotide,which is shown to have anti -HCMV activity [31].
6.2 Aptamers
Oligonucleic acid or peptide molecules which bind to a specific target molecule are known as Aptamers. Inhibitors of HCV-NS3 helicase [31] and HCV NS-3 proteinase [32] have been developed using this approach. The major problem with this approach is the problem in delivery that needs to be addressed. Another new approach involves the use of peptide aptamers to treat viral infections. The proteins which are designed to interfere with other protein interactions inside cells are known as peptide aptamers. They consist of a variable peptide loop attached at both ends to a protein scaffold.it has been shown that out of the several peptide aptamers capable of binding to HBV core protein,one of them can inhibit viralfunction such as capsid formation,replication and virion formation [33].
6.3 Ribozymes
Ribozymes also called as RNA enzymes are RNA molecules that catalyze a reaction. These bring about the hydrolysis of one of its own phosphodiester bonds or of any other RNA. In some cases ribozymes have been shown to be very effective in inhibiting viral replication. The use of ribozymes as the therapeutic agents is important at low level viral gene expression as in the case of latent infection. It is very difficult to treat the viral infection using ribozyme in the case when the viral gene expression is fully activated [34].
7. Future Prospects
In last few years 5 new antiviral drugs have been licensed. Now the total number of antiviral drugs developed is increased to 42. All this can be attributed to the greater understanding of the viral life cycle facilitated by the genome sequencing projects and the emerging new technologies. More over the in silico resources have also proved to be very promising for the drug discovery. Recent advances in the field of gene molecular biology like gene inhibition by antisense and siRNA revolutionized the validation of a large number of ideal drug targets identified by either DNA micro arrays or by any other method. More over the siRNA screening of the whole genome will do complete validation and evaluation of host gene products for viral replication. These approaches thus represent an unparalleled prospect for characterizing the functional host gene products that are essential for virus replication and disease [35,36,37]. Since in the last few decades the emergences of new infectious diseases are continuously increasing,which is a serious problem of concern for the public health. Till date there is no approved antiviral therapy for any of the emerging or re-emerging viral diseases. However the combined efforts of biology and chemistry in drug discovery show some promising results for therapeutic management of these diseases [26]. It is very important to counteract the development of resistance in the viruses against the antiviral drugs,which may be done by targeting the conserved proteins of the viruses.
8. Conclusions
There has been tremendous progress in understanding of molecular mechanisms and genetic basis of diseases in the recent years. Many new drugs have been developed and a lot more are in the process of development. However,the emerging new infectious diseases remain a challenge. Moreover,the failure of drugs in human trials is also a common phenomenon that needs to be addressed and worked out. Many new technologies have emerged that are expected to show promising results.
The battle between human and the viruses is on,with both of them rapidly improving their attacking and defense strategies. The growing knowledge about viruses and the rapidly evolving tools and techniques is certainly going to help us in the development of new antiviral drugs. As we are able to understand the viruses better,it will be possible to develop effective measures for combating the viral diseases,and the researchers all over the world are trying their best that this time comes soon and we live in the world which is free from viral diseases.
Acknowledgements
Authors are grateful to Council of Scientific and Industrial Research (CSIR),India and Director,Centre for Cellular and Molecular Biology,for the encouragement and support for this work. S.K.S. and M.P.N. are supported by NIH (USA) MERIT Award R37DA025576.
References
- Nene Y.L. (2007) A glimpse at viral diseases in ancient period. Asian Agri-History,11(1): 35-46.
- Nelson K.E.,Williams C.F. (2001) Early history of infectious diseases (Chapter 1). In Infectious Disease Epidemiology. Theory and Practice (Nelson K.E.,Williams C.F.,Graham N.MH editors),Jones and Bartlett Publishers,Sudbury,MA (ISBN0763728799),pp. 1-29.
- Washington,D.C.,Jan (2000) In National Intelligence Council (NIC),“The global infectious disease threat and its implications for the United States,” NIE-99-17D,(https://www.fas.org/irp/threat/nie99-17d.htm; accessed on Feb 4,2010).
- World Health Organization South-East Asia Regional Office. (2005) Combating emerging infectious diseases (SEA-CD-139) (https://www.searo.who.int/LinkFiles/Avian_Flu_combat ing_emerging_diseases.pdf; accessed on Feb 4,2010).
- Bean B. (1992) Antiviral therapy: current concepts and practices. Clinical Microbiology Reviews,5(2): 146- 182.
- Field H.J.,De Clercq E. (2004) Antiviral Drugs – a short history of their discovery and development. Microbiology Today,31: 58–61.
- Dolan K.A.,Moukheiber Z. (2003) The golden age of antiviral drugs. (https://www.forbes.com/global/2003/1027/090.html; accessed on Feb 4,2010).
- De Clercq E.,Brancale A.,Hodge A.V.,Field H.J. (2006) Antiviral Chemistry & Chemotherapy's current antiviral agents Fact File (1st edition). Antiviral Chemistry Chemotherapy,17(3): 113-166.
- De Clercq E.,Field H.J. (2008) Antiviral Chemistry & Chemotherapy's current antiviral agents Fact File (2nd edition): retroviruses and hepadnaviruses. Antiviral Chemistry Chemotherapy,19(2): 75-105.
- Abel S.,Back D.J.,Vourvahis M. (2009) Maraviroc: pharmacokinetics and drug Interactions. Antiviral Therapy,14(5): 607-618.
- Emmelkamp J.M.,Rockstroh J.K. (2007) CCR5 antagonists: comparison of efficacy,side effects,pharmacokinetics and interactions. European Journal of Medical Research,12(9): 409-417.
- Ghosh A.K.,Dawson Z.L.,Mitsuya H. (2007) Darunavir,a conceptually new HIV-1 protease inhibitor for the treatment of drug-resistant HIV. Bioorganic & Medicinal Chemistry,15(24): 7576-7580.
- Doyon L.,Tremblay S.,Bourgon L.,et al. (2005) Selection and characterization of HIV-1 showing reduced susceptibility to the non-peptidic protease inhibitor tipranavir. Antiviral Research,68(1): 27-35.
- Balfour H.H.,Jr.,Hokanson K.M.,Schacherer R.M.,et al. (2007) A virologic pilot study of valacyclovir in infectious mononucleosis. Journal of Clinical Virology,39(1): 16-21.
- Lazonick W. (2009) How American capitalism really works: some lessons for developing countries. (https://vlex.com/vid/73809681; accessed on Feb 4,2010).
- Buchanan L.,Jurek P.,Redshaw R. (2007) Nuclear imaging drug development tools. PET and SPECT help enhance the quality of a product and prevent late-stage failure,27(8): (https://www.genengnews.com/articles/chtitem.aspx?tid =2073; accessed on Feb 4,2010).
- Baranczewski P.,StaÃÆââ¬Â¦Ãâââ¬Å¾czak A.,Sundberg K.,et al. (2006) Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacological Reports,58(4): 453-472.
- Jones P.S. (1998) Strategies for antiviral drug discovery. Antiviral Chemistry & Chemotherapy,9(4): 283-302.
- American Federation for Aging Research. (2007) Drug Discovery and Development,(https://websites.afar.org/site/PageServer?pagename=I A_RandD; accessed on Feb 4,2010).
- Zhu H.,Cong J.P.,Mamtora G.,et al. (1998) Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proceedings of the National Academy of Sciences USA,95(24): 14470-14475.
- Jain K.K. (2005) Applications of AmpliChip CYP450. Molecular Diagnosis,9(3): 119-127.
- Foundation for the National Institutes of Health. (2007) In The Biomarkers Consortium,(https://www.biomarkersconsortium.org/index.php?optio n=com_content&task=view&id=51&Itemid=61; accessed on Feb 4,2010).
- Kapetanovic I.M. (2008) Computer aided drug discovery and development (CADDD): in silicochemico biological approach. Chemico-Biological Interactions,171(2): 165-176.
- Yuan H.,Parrill A.L. (2000) QSAR development to describe HIV-1 integrase inhibition. Journal of Molecular Structure: Theochem,529(1-3): 273-282.
- Gershell L.J.,Atkins J.H. (2003) A brief history of novel drug discovery technologies. Nature Reviews Drug Discovery,2(4): 321-327.
- Neefjes J.,Dantuma N.P. (2004) Fluorescent probes for proteolysis: Tools for drug discovery. Nature Reviews Drug Discovery,3(1): 58-69.
- Aamnoutse R.E.,Verweij-van Wissen C.P.,Underberg W.J.,et al. (2001) High-performance liquid chromatography of HIV protease inhibitors in human biological matrices. Journal of Chromatography B: Biomedical Sciences and Applications,764(1-2): 363- 384.
- Nikolin B.,ImamoviÃÆââ¬Å¾Ãâââ¬Â¡ B.,MedanhodziÃÆââ¬Å¾Ãâââ¬Â¡-Vuk S.,Sober M. (2004) High perfomance liquid chromatography in pharmaceutical analyses. Bosnian Journal of Basic Medical Sciences,4(2): 5-9.
- Erlich K.S.,Mills J.,Chatis P.,et al. (1989) Acyclovirresistant herpes simplex virus infections in patients with the acquired immunodeficiency. New England Journal of Medicine,320(5): 293-296.
- Quan Y.,Rong L.,Liang C.,Wainberg M.A. (1999) Reverse transcriptase inhibitors can selectively block the synthesis of differently sized viral DNA transcripts incells acutely infected with human immunodeficiency virus type 1. Journal of Virology,73(8): 6700-6707.
- Kumar P.K.,Machida K.,Urvil P.T.,et al. (1997) Isolation of RNA aptamers specific to the NS3 protein of hepatitis C virus from a pool of completely random RNA. Virology,237(2): 270–282.
- Urvil P.T.,Kakiuchi N.,Zhou D.M.,et al. (1997) Selection of RNA aptamers that bind specifically to the NS3 protease of hepatitis C virus. European Journal of Biochemistry,248(1): 130–138.
- Crawford M.,Woodman R.,Ko Ferrigno P. (2003) Peptide aptamers: tools for biology and drug discovery. Briefings in Functional Genomics & Proteomics,2(1): 72-79.
- Sczakiel G.,Nedbal W. (1995) The potential of ribozymes as antiviral agents. Trends in Microbiology,3(6): 213-217.
- Tseng C.K. (2005) Overview of antiviral drug discovery and development. In Antiviral Drug Discovery for Emerging Diseases and Bioterrorism Threats (Torrence P.F.),John Wiley & Sons: New York,pp. 31−82.
- Saxena S.K.,Mishra N.,Saxena R. (2009) Advances in antiviral drug discovery and development: Part I: Advancements in antiviral drug discovery. Future Virology,4(2): 101−107.
- Saxena S.K.,Mishra N.,Saxena R. (2009) Advances in antiviral drug discovery and development: Part II: Advancements in antiviral drug development. Future Virology,4(3): 209−215.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences