Cd2+ Biosorption by Pretreatment Biomass of Highly Cadmium Resistant Fungus Humicola sp
Tinnapan Netpae
Faculty of Science and Technology, Nakhon Sawan Rajabhat University, Thailand.
- Corresponding Author:
- Tel: +66 (0)5621 9100
Fax: +66 (0)5622 1237
E-mail: tinnapan_net@yahoo.com
Abstract
This research was carried out to estimate the removal of Cd2+ from an aqueous solution by pretreatment of Humicola sp. biomass with acid (H2SO4) and base (NaOH). The influence of different treatments of dried Humicola sp. biomass on its Cd2+ biosorption activity, indicated that 10% NaOH was increased biosorption of Cd2+ in comparison with the untreated and acid treated biomasses. Maximum Cd2+ biosorption of pretreated biomass by base took place at initial solution at pH 6 and 7 after 150 minutes, while the maximum adsorption of Cd2+ in pretreated biomass by acid was obtained highest at pH 6 after 60 minutes. Cadmium ions removal increase within the temperature has raised more than 40 oC. Desorption experiments indicate that the desorption efficiency with 0.1 M HNO3 solution reaches 96.35% and 83.32% in acid and base treatment, respectively.
Keywords
Humicola sp.; pretreatment; biosorption; cadmium.
1. Introduction
Cadmium (Cd) is extremely toxic, the problem of Cd contamination occurs when aqueous effluents from many industrial processes that contain dissolved heavy metals without treatment are disposed. They may have an adverse impact on the environment. Various types of microorganism including bacteria, algae, yeast and fungi are capable of adsorbing Cd from solution due to metal attracting compositions or natively charged functional groups on their cell wall which is mainly binding properties of such microorganism. Humicola sp. fungus is able to grow in the sediments of highly contaminate with Cd [1]. Recent studies have identified the potential for Cd2+ biosorption by Humicola sp. of viable and non-viable biomass pretreated by heating while Cd concentration were increased in the same pattern, but viable biomass showed better Cd2+ removal ability than non-viable biomass [2]. Although living cell showed better Cd2+ removal ability than dead cell, but the use of living cells have many disadvantages as they are more sensitive to Cd concentration, toxicity effect and adverse conditions such as pH and temperature. Moreover, they require nutrient supply and culture maintenance, which cause of increasing of operating cost. According to many workers, the chemical pretreatment methods may provide better results in the list of number of pretreatment protocols owing to change in cell wall chemistry of the biosorbent [3-5].
In this research, adsorption ability non-viable biomass of Humicola sp. pretreated by acid (HNO3) and base (NaOH) were investigated for removal of Cd from aqueous solution under experimental conditions.
2. Materials and Methods
Microorganism
Humicola sp. was isolated from sediment in Mae Tao creek in Mae Sot District, Tak Province, Thailand. Fungal spores were obtained from a 5 day old culture grown on Potato Dextrose Agar (PDA) at 30±2° C. The spores were collected in 0.01 % tween-80 solution.
Biomass preparation
Humicola sp. biomass was cultivated in Potato Dextrose Broth (PDB), using the shake flask method. Spore suspensions (1x108 spores) were cultivated in 250 ml erlenmeyer flask with 50 ml PDB. Once inoculated, flasks were shaken on a rotary shaker at a speed of 150 rpm for 3 days at 30±2 ° C. Harvested biomasses (20 g wet wt.) were pretreated with NaOH and HNO3 by suspending the biomass in 50 ml of 10% NaOH and HNO3 for 30 minutes at 30±2 ° C. The biomass were collected and washed many times with distilled water until the pH of supernatant became 7. Finally, pretreated biomass was dead in an autoclaved at 121oC for 20 minutes and then harvested by filtering through a membrane filter and dried at 80 °C in an oven for 12 hours. This was then ground, using a blender to break cell aggregates into smaller fragments. The biomass was then passed through 100 μm mesh sieve of to obtain particle sizes of less than 0.5 - 1.0 mm diameter. The pretreatment biomass was preserved in airtight jar to be used in the Cd2+ uptake studies.
Batch isotherm experiments
The isotherm experiments were studied using 0.1 g of cell dried. Biosorbent was suspended in 50 ml of metal solution and initial Cd concentrations were varied over the range 0 to 150 mg 1-1. The working solutions adjusted to pH 5 were shaken at 150 rpm for 2 hours at 30±1 ° C. Samples were taken at time intervals, centrifuged and then analyzed for residual metal concentration.
The adsorption capacity, q, is calculated from the difference between initial and equilibrium concentrations as shown in equation:
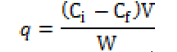
where q is the Cd uptake (mg g-1 dry wt.), Ci and Cf are the initial and equilibrium concentration of metal solution (mg l-1), W is the adsorbent dosage (g), and V is the volume of the Cd-bearing solution contacted with sorbent (ml). Several adsorption isotherms originally used for gas phase adsorption are available and rapidly adopted to correlated adsorption equilibrium in heavy metal adsorption. The most widely use among them is Lanmiur equation. The resulting values of Cf / q were plotted against Ci to obtain a Langmuir plot typical of the sorption behavior [6].
Effect of temperature, pH and contact time on Cd removal by fungus
In order to evaluate the effect of temperature, pH and contact time on the Cd2+ uptake, the experiment was conducted in the same manner, except the temperature of Cadmium solution was changed to 30, 40, 50, 60 and 70 oC. The pH of the solution was prepared to be in the range between 3.0 and 9.0 before mixing biomass. The pH was adjusted to the required value with 0.1M NaOH or 0.1M HNO3. The period of contact time was studied up to 180 min by using the procedure described earlier, samples were collected every 30 min (30, 60, 90, 120, and 180 min, respectively).
Cd desorption experiments
The 0.1M HNO3 solution was used to elute Cd2+ from both biomass. Following the Cd sorption experiments, the Cd-loaded biomass was prepared by centrifugation, washed and returned to 25 ml of the effluent 0.1 M HNO3 for 30 min on a rotary shaker (125 rpm). Metal concentrations were determined after separating the biomass from eluting agent by filtration.
Atomic absorption analysis
Cd remaining in solutions was analyzed by using an atomic absorption spectrophotometer (Variance spectra model AA-220 FS) with and oxidizing flame of an air acetylene mixture at a ratio of air flow at 13.50 l min-1 and acetylene flow 2 l min-1.
Statistical analysis
All the experiments were triplicate. Mean values were used in the analysis of data by using the analysis of variance (one - way ANOVA) and Post Hoc. Duncan test (p<0.05).
3. Results and Discussion
Uptake Mechanism of Cd by biomass pretreated with acid and base
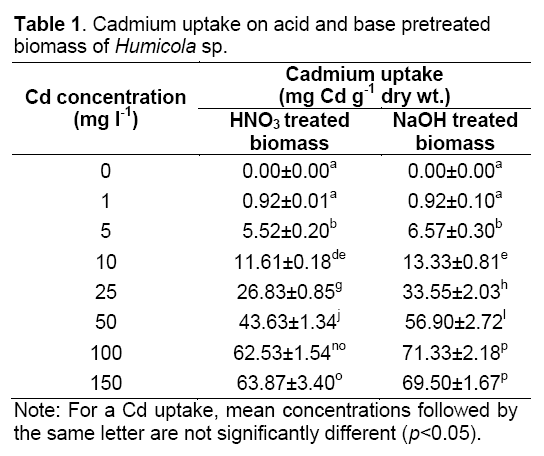
Pretreatment of Humicola sp. biomass with H2SO4 and NaOH were proved to increase or maintain adsorption efficiency and capacity in comparison to untreated biomass. At Cd concentration of 100 mg l-1, acid and base pretreatment biomass removed Cd of 62.53±1.54 mg Cd g-1 dry wt. and 71.33±2.18 mg Cd g-1 dry wt., respectively. The Cd2+ uptake by pretreated biomass was showed in the Table 1. This value is better than untreated of biomass because the maximum removal extent of viable biomass of Humicola sp. is 61.77±3.25 mg Cd g-1 dry wt., under identical conditions [2].
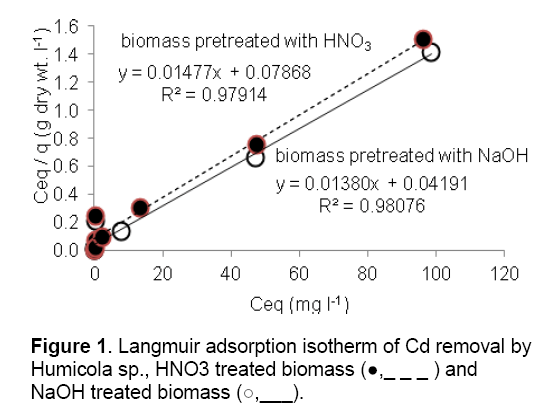
The adsorption performance of the Cd2+ biosorbent can be described by mathematical model in term of Langmuir isotherm. These isotherms follow the typical Langmuir adsorption pattern as shown by the linear transformation (Figure 1). The linearised form of Langmuir equation is represented by the following expression:
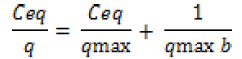
where Ceq is the the equilibrium solution concentration (mg l-1), q max is the amount adsorbed at equilibrium (mg g-1), the Langmuir constants qmax and b are related to adsorption capacity and energy of adsorption, respectively [7]. The linear plot between Ceq/q with Ceq shows that investigated metal ions adsorption by pretreated biomass. As compared in Table 2, the base pretreated biomass has a greater capacity (qmax) than acid pretreated biomass and untreated biomass for Cd adsorption. Conversely, the untreated Humicola sp. biomass has higher binding constant than the pretreated biomass.
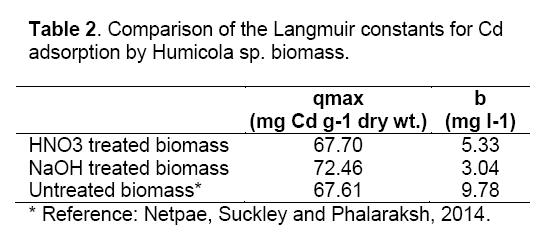
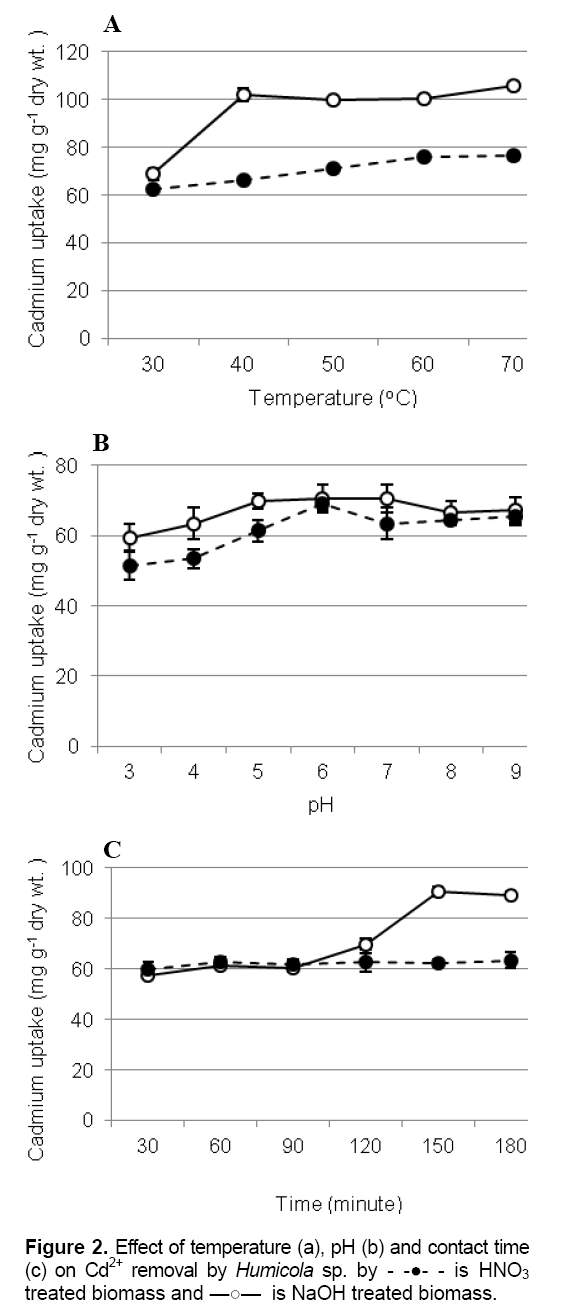
Effect of temperature, pH and contact time on Cd removal
Temperature and pH were the most important facter influencing the biosorption process. Under the same condition, the base pretreatment biomass was high accumulation than biomass pretreated by acid. The binding of Cd2+ to pretreated biomasses were enhanced when temperature increases. From the results, Cd2+ removal increase rapidly within the temperature has raised more than 40 oC in base pretreated biomass while the biosorption by acid pretreated biomass increased slowly during the temperature increase (Figure 2A). Higher temperatures usually enhance sorption as a result of the increased surface activity and kinetic energy of the solute [11]. However, physical damage to the biosorbent can be expected at higher temperatures [12].
Biosorption of pretreatment of Humicola sp. biomass with H2SO4 and NaOH have the similar trend that the pH of the metal solution increase from 3 to 9. The accumulation level was found the optimum pH 6 to 7. Accumulations at extreme pH 2-4 were rare (Figure 2B). Other studies showed that increasing of heavy metal biosorption capacity of pretreated fungi occurred as pH increased [13] [14]. Optimum contact time for Cd adsorption by Alkaline biomass treatment by NaOH was 150 minutes, while the rate of biosorption by biomass pretreated with acid was faster and contributed significantly (p< 0.05) to equilibrium uptake 99.14 % recovery being achieved within 60 minutes (Figure 2C).
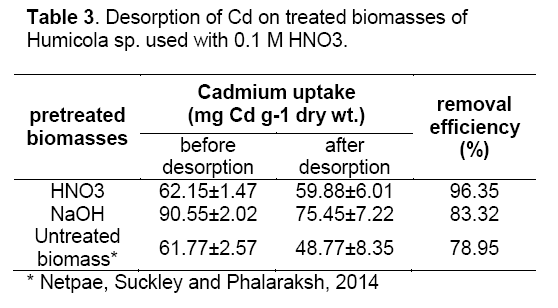
Cadmium desorption
0.1M nitric acid solution was able to effectively elute the biosorbed Cd2+ (Table 3). The Removal efficiency of Cd2+ was decreased by about 96.35% and 83.32% in acid and base treatment respectively. The decrease in Cd2+ uptake by acid desorbent might be due to the increase of the concentrations of competing hydronium ions. It is also possible that the physical structure of the biomass becomes damaged by this acid [15].
4. Conclusions
According to the results of this research, it is obvious that the pretreatment biomass of Humicola sp. by acid (HNO3) and base (NaOH) are able to remove Cd2+ from aqueous solution. The base treated biomass of Humicola sp. can give rise the higher Cd2+ removal efficiency in solution than untreated and acid treated biomasses. Maximum Cd2+ biosorption of pretreated biomass by base took place at initial solution at pH 6 and 7 after 150 minutes. The use of nitric acid as eluting agent is effective for desorbing of Cd2+ loaded biomass.
Acknowledgments
Financial support from Nakhon Sawan Rajabhat University, Thailand is gratefully acknowledged.
References
- Netpae T., Suckley S., Phalaraksh C. (2014) Biosorption of Cd2+ from Aqueous Solutions by Tolerant Fungus Humicola sp. Advances in Environmental Biology, 8(21): 308-312.
- Netpae T., Suckley S., Phalaraksh C. (2015) Cadmium Tolerance Fungi Isolated from Polluted Sites in the Mae Tao creek, Thailand. Advanced Studies in Biology, 7(1): 29-37.
- Das N., Charumathi D., Vimala R. (2007) Effect of pretreatment on Cd2+ biosorption by mycelia biomass of Pleurotus florida. African Journal of Biotechnology. 6(22): 2555-2558.
- Wang J., Chen C. (2009) Biosorbents for heavy metals removal and their future. Biotechnology Advances. 27: 195-226.
- Javaid A., Bajwa R.. Manzoor T. (2011) Biosorption of heavy metals by pretreated biomass of Aspergillus niger. Pakistan Journal of Botany., 43(1): 419-425.
- Langmuir I. (1916)The constitution and fundamental properties of solids and liquids, Part. I: Solids. Journal of the American Chemical Society, 38: 2221.
- Ahalya N., Kanamadi R.D., Ramachandra, T.V. (2006) Biosorption of Iron (III) from aqueous solution using the husk of Cicer arientinum. Indian Journal of Chemistry Technology. 13: 122 -127.
- Mameri N., Boudries N., Addour L., Belhocine D., Lounici H., Grib H.,Pauss A. (1999) Batch zinc biosorption by a bacterial nonliving Streptomyces rimosus biomass. Water Research., 33: 1347-1354.
- Dhankhar R., Hooda A. (2011) Fungal biosorption – an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environmental Technology. 32(5): 467-491.
- Rao P.R., Bhargavi C. (2013) Studies on biosorption of heavy metals using pretreated biomass of fungal species. International Journal of Chemistry and Chemical Engineering. 3(3): 171-180.
- Joshi J., Sahu O. (2014). Adsorption of Heavy Metals by Biomass. Journal of Applied & Environmental Microbiology, 2 (1): 23-27.
- Pimpa W., Netpae T.,(2004) Use of pelletted biomass of Aspergillus oryzae for lead removal. Thai Environmental Engineering Journal, 18(1): 21-28.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
 and NaOH treated biomass
and NaOH treated biomass 
 is HNO3 treated biomass and
is HNO3 treated biomass and  is NaOH treated biomass.
is NaOH treated biomass.