Bodyweight-forearm Ratio, Cranial Morphology and Call Frequency Relate to Prey Selection in Insectivorous Bats
Robbie Weterings, Chanin Umponstira
Robbie Weterings1,2,*, Chanin Umponstira1
1Faculty of Agriculture Natural Resources and Environment, Naresuan University, Muang Phitsanulok, 65000 Thailand
2Cat Drop Foundation, Boorn 45, 9204 AZ, Drachten, The Netherlands
- Corresponding Author:
- Tel: +66(0)890176087
E-mail: r.weterings@catdropfoundation.org
Abstract
The feeding habits of insectivorous bats are of great interest to people, because they are considered to be important in the control of insect pests. Here we present a study showing the relationship of bat morphology to differences in prey selection by various bat species. We compared dietary data from 10,884 faecal pellets, bodyweight, cranial length, forearm length and echolocation calls from published peer-reviewed studies for 92 bat species. We demonstrated that insectivorous bats tend to prefer certain insect orders which we have grouped as soft bodied insects, hard bodied insects and Lepidoptera. Wing characteristics which we measured by bodyweight-forearm ratio showed the strongest relationship with hard insects followed by longest cranial length. The content of soft insects in bat diets was negatively related to bodyweight, forearm length and longest cranial length. Lepidoptera content was positively related to the echolocation frequency with the maximum intensity (FMAXE), bats with high FMAXE fed on more Lepidoptera than those with low frequencies. We propose that a combination of dietary analysis and morphological analysis is needed to make strong inference about prey preference rather than comparing the dietary analysis with the insect abundance at the location were the bat or faecal pellets were collected
Keywords
Chiroptera; diet; cranial morphology; bodyweight-forearm ratio; Echolocation.
Introduction
The feeding habits of insectivorous bats are of great interest to people, because they are considered to be important in the control of insects ranging from agricultural pests such as moths (Lepidoptera) [1,2] to disease vectors such as mosquitoes (Culicidae) [3,4]. A thorough understanding of feeding habits and prey preference is still lacking regardless of the several hundred dietary analyses that have been conducted and published over the last few decades. Prey preference is difficult to determine in the field due to the large distances that bats fly to their foraging habitats [5]. Analysing faecal pellets or stomach content does give an understanding of what the species is eating, but there are no studies known in which diet has been compared to the actual food supply. Many studies compare the dietary analysis with the insect abundance at the location were the bat or faecal pellets were collected, but this is in most cases not the location were the bat is foraging [6].
A few studies have combined the data from dietary analysis of many species to look for patterns related to biological characteristics such as skull morphology, wing span, bodyweight and/or echolocation which can help us predict the feeding habits and prey preference [6-8]. Food hardness, for example, is related to the bite force [8,10,11], which depends on skull morphology [12,13] and relates to body size or weight [14]. Wing morphology influences feeding habits, it having been shown that different wing types cause different flight styles affecting agility and thus hunting behaviour [15]. Bogdanowicz and colleagues [9] showed that the peak frequency (frequency of maximum intensity) of echolocation is also strongly related to different prey types. When peak frequencies become higher the amount of Lepidoptera in the diet increases; however, when the frequencies get lower, the amount of Coleoptera increases. Bats that use higher call frequencies are capable of detecting much smaller insects in comparison to bats that use low frequencies following the general physics of acoustics [16,17]. The wavelength has to be shorter than the size of an insect for a bat to be able to detect the prey [17]. The small differences in skull morphology, flight style and echolocation have made bats very successful in many parts of the world because they can exploit a wide range of niches [18].
Here we present a study of the relative importance of different morphological characteristics in determining the dietary content of different insect types. We first conducted a comprehensive analysis of the co-occurrence of food items in the diets of insectivorous bat species. The content of ten insect orders in the diets of 92 bat species were compared. These data were acquired from existing peer-reviewed studies. We expected to find two clusters of insect orders; one with hard bodied insects and one with softer insects [8,10,11]. Finally we used these co-occurring insect orders to assess how different morphological characteristics are affecting prey selection in bats. We expected that body size, echolocation, skull size and forearm length are important factors that determine the feeding behaviour [7-9]. Diets for single bat species can differ tremendously between seasons and locality [2,19]. Therefore, we expected to find large variation in our dataset that could not be explained by these morphological characteristics. In comparison to previous studies, this study does not focus on a narrow range of food items, but includes an analysis of the complete diet of bats.
2. Methods
Data collection
We analysed dietary content, bodyweight, longest cranial length, forearm length and echolocation calls based on data from published peer-reviewed studies. Data regarding diets were only considered when food items were presented as volume percentage and were based on faecal pellets. We excluded studies based on stomach content to avoid biases caused by using two different methods. Insectivorous bats digest their food very rapidly and do not thoroughly chew their prey. Therefore, faecal pellets often contain many parts of prey items that can still be used to identify the prey to the taxonomic order and sometimes to higher taxonomic levels [6,20]. When dietary data for multiple species were available, we calculated a weighted mean in which the number of studied pellets was used as weight. The final dataset consisted of 31 food categories: 25 insect orders, spiders (Araneae), two insect families, fish, plants and unidentified. These categories were later reduced to the ten most common invertebrate orders. For echolocation data we only considered the frequency with the maximum energy (FMAXE). Data regarding bodyweight, longest cranial length and forearm length were also acquired from published data. We used forearm length and longest cranial length as variables representing wing and skull morphology, because these are most often documented in existing literature When multiple values for the same species were encountered, we calculated the mean.
Nonmetric multidimensional scaling
We used Non-metric Multidimensional Scaling (NMS) based on Euclidean distances as a tool for creating ordination plots. The goodness of fit of a NMS can be assessed through the stress-values. A stress-values of 0.0 to 0.1 is considered a good fit, a stress value of 0.1 to 0.2 is considered a moderate fit and a higher stress values are considered to be a poor fit [21]. We used dietary content of different insects as our input variables to assess the similarity of bat species based on their diets. Only those insects orders were used that were most common. This included all orders that occurred in the diet of 20 or more bat species. First these food items were plotted for all 92 species. Then we added grouping polygons based on the standard error of the weighted average. These polygons displayed the dominant food item based on three categories: hard bodied insects, soft bodied insects and Lepidoptera. Hard bodied insects included Coleoptera, Hemiptera, Homoptera and Orthoptera, while soft bodied insects included Diptera, Trichoptera, Neuroptera and Araneae. A category was considered dominant when the percentage was highest for this group in comparison to the other two categories. We also added polygons that represented the four most common bat genera in our dataset: Pippistrellus (n=8), Myotis (n=24), Hipposideros (n=6) and Rhinolophus (n=12). We compared the differences of bodyweight, longest cranial length, forearm length and FMAXE between these four genera using an ANOVA procedure with a multiple comparison test based on the Tukey-Kramer test proposed by Herberich et al. (2010). This procedure allows data to be heteroscedastic and unbalanced [22]. Where necessary, data were log-transformed to obtain normality. All analyses were conducted with RStudio version 0.96.331 [23] built on R version 3.0.1 [24]. The 'vegan' package was used for NMS [25], while the 'sandwich' package was used for the multi-comparison procedure [26].
Generalized Linear Models
Food habits were modelled using weighted Generalized Linear Model (GLM) assuming a Tweedie compound Poisson-gamma distribution and a log-link function [27]. The Tweedie distribution does not assume a constant variance, but relates the variance to the mean following the power law [27,28]. We used this distribution, because the data were following a Poisson shaped distribution but are continuous rather than count data. The Tweedie compound Poisson-gamma distribution allowed us to avoid any transformations of the response variables, which made it easier to compare the different models. All variables were separately modelled because of collinearity among explanatory variables. We did not want to exclude any variable or use a factor or dummy variable because we were interested in the relationship of each separate variable with the diet. Collinearity among explanatory variables was visualized in scatterplots to which we added fitted lines and confidence intervals based on generalized additive models using local regression smoothers. The weights that were included in the models were based on the number of pellets investigated for each bat species. The total dataset was based on a number of 10,884 faecal pellets. Prior to the analysis, all variables were standardized to a mean of zero and a standard deviation of one [29]. The 'tweedie' package [30] was used for fitting the generalized linear models.
3. Results
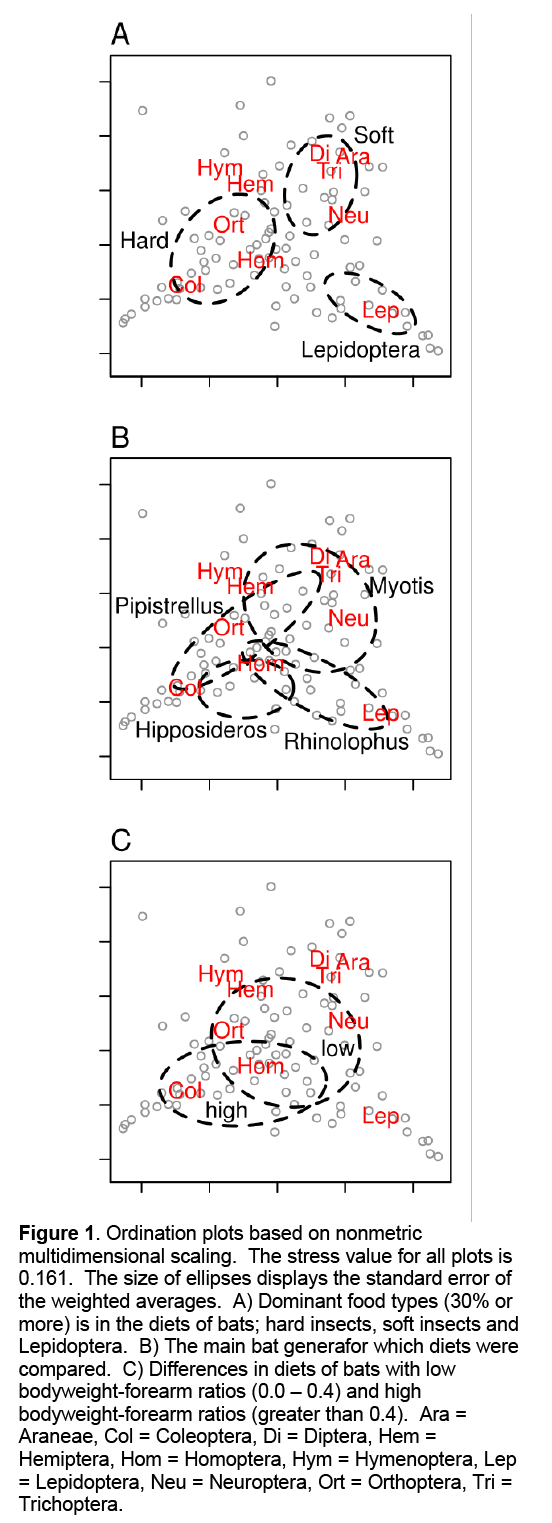
The NMS ordination of all main insect orders clearly showed that certain food items in bat faeces often co-occur (Figure 1A). Coleoptera, Hemiptera and Homoptera (hard bodied) were often found in faeces together. Pipistrellus and Hipposideros species tended to feed more often on these harder insects (Figure 1B). Soft bodied prey e.g. Diptera, Araneae and Trichoptera, were more often found in the faeces of other bat species. Lepidoptera deviated strongly from all the other insect orders and were not commonly found with other food items. Myotis species fed on considerably softer insects. Rhinolophus species fed most on Lepidoptera in comparison to the other three genera, while Pipistrellus species fed the least on Lepidoptera. Pipistrellus species were significantly smaller than species from the other three genera (Table 1) which was also reflected in forearm length and the longest cranial length. Differences in bodyweight between the other genera were not significant. Rhinolophus and Hipposideros species had significant higher FMAXE compared to Pipistrellus and Myotis species. Rhinolophus species had generally a lower but non-significant bodyweight than Hipposideros species, however, this was not reflected in the longest cranial length. Bat species with low bodyweight-forearm ratios (e.g. Pipistrellus) generally fed on softer insects in comparison to species with high bodyweight-forearm ratio (e.g. Hipposideros, Figure 1C).
Figure 1: Ordination plots based on nonmetric multidimensional scaling. The stress value for all plots is 0.161. The size of ellipses displays the standard error of the weighted averages. A) Dominant food types (30% or more) is in the diets of bats; hard insects, soft insects and Lepidoptera. B) The main bat generafor which diets were compared. C) Differences in diets of bats with low bodyweight-forearm ratios (0.0 – 0.4) and high bodyweight-forearm ratios (greater than 0.4). Ara = Araneae, Col = Coleoptera, Di = Diptera, Hem = Hemiptera, Hom = Homoptera, Hym = Hymenoptera, Lep = Lepidoptera, Neu = Neuroptera, Ort = Orthoptera, Tri = Trichoptera.
The GLMs how that the content of hard insects in diets is related to the bodyweight, cranial length, forearm length, bodyweight-forearm ratio and call frequency. Bodyweight-forearm ratio showed the strongest relationship with hard insects followed by longest cranial length (Table 2). Larger bats with relatively large skulls where most likely to have the highest content of hard insects in their diet. The FMAXE was negatively related to the content of hard insects in the diet. Nevertheless, the model based on FMAXE scored only slightly better than the null model. The content of soft insects in bat diets was related to bodyweight, forearm length and longest cranial length but not to FMAXE. Cranial length showed the strongest negative relationship to soft insect content. Smaller bats with relatively small skulls were most likely to have high amounts of soft insects in their diet. Lepidoptera content was positively related to FMAXE, bats with high FMAXE fed on more Lepidoptera than those with low frequencies. Lepidoptera content was not related to any of the other variables.
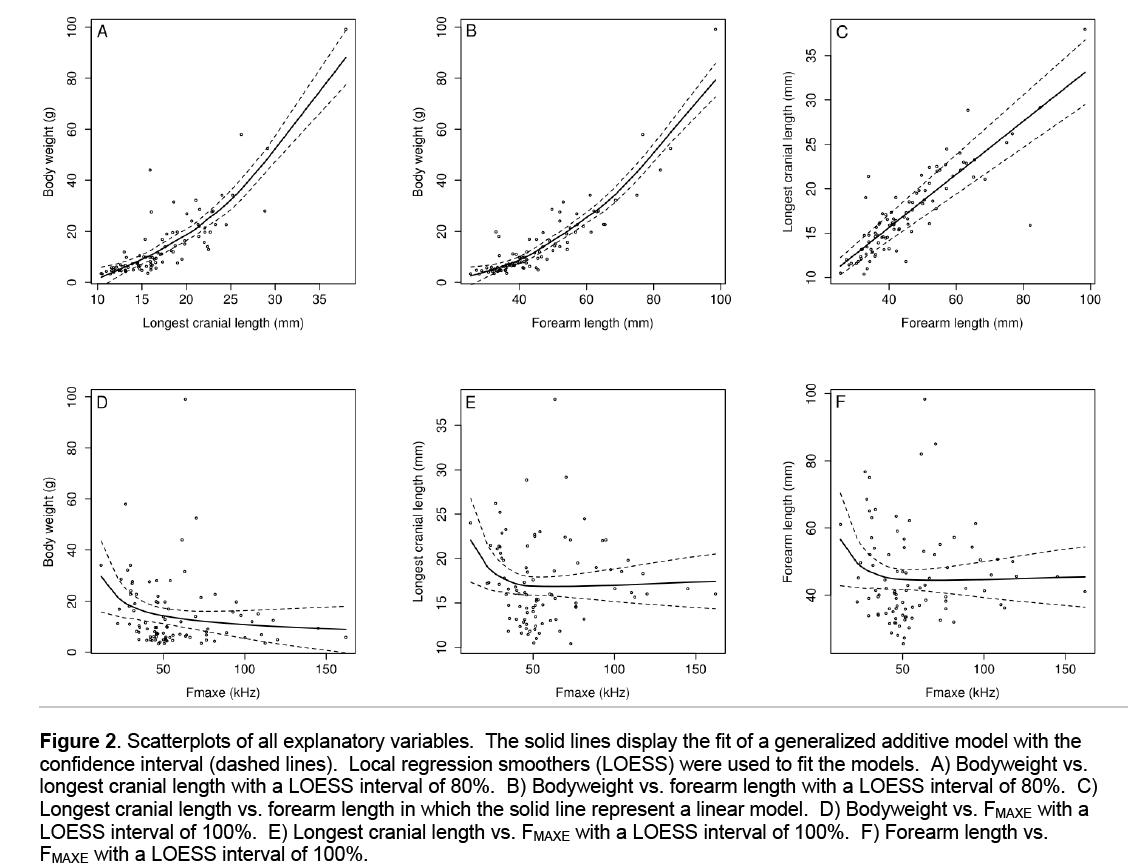
Bodyweight showed a non-linear relationship with longest cranial length (F = 19.16, d.f. = 88.3, P < 0.001, Figure 2) and forearm length (F = 33.4, d.f. = 88.3, P < 0.001). Cranial length and forearm length were showing a strong linear relationship (F = 262.7, d.f. = 90, P < 0.001). Larger bats generally had lower peak frequencies although this non linear relationship was not significant (F = 2.64, d.f. = 89.5, P = 0.118). There was a weak non linear relationship between FMAXE and cranial length (F = 9.75, d.f. = 89.5, P = 0.012) and between FMAXE and forearm length (F =5.62, d.f. = 89.5, P = 0.041).
Figure 2: Scatterplots of all explanatory variables. The solid lines display the fit of a generalized additive model with the confidence interval (dashed lines). Local regression smoothers (LOESS) were used to fit the models. A) Bodyweight vs. longest cranial length with a LOESS interval of 80%. B) Bodyweight vs. forearm length with a LOESS interval of 80%. C) Longest cranial length vs. forearm length in which the solid line represent a linear model. D) Bodyweight vs. FMAXE with a LOESS interval of 100%. E) Longest cranial length vs. FMAXE with a LOESS interval of 100%. F) Forearm length vs. FMAXE with a LOESS interval of 100%.
4. Discussion
We demonstrated that insectivorous bats tend to prefer certain invertebrate orders which we have grouped as soft bodied invertebrates, hard bodied insects and Lepidoptera. Although bats do specialize and prefer certain prey, they do not exclude other prey from their diets. Preference of certain prey items can be explained by certain characteristics of the bats. The most important characteristics are bodyweight-forearm ratio, cranial length and FMAXE. Bodyweight-forearm ratio was most important in predicting the content of hard insects, the longest cranial length was most important for soft insects and FMAXE was most important for Lepidoptera. The differentiation of these characteristics in bats is likely to have evolved from trophic specialization [31,32]; as is hypothesized for other species [33,34]. Where several species of bats co-occur, it is in the interest of the individual species to specialize on prey that are most abundant or are not available for competitors [34,35]. Differences in flight style, cranial morphology and echolocation could therefore have found their origin within resource partitioning [36].
Wing morphology is really important in shaping the flight style e.g. aerial-hawking, trawling, gleaning or perch hunting [37]. Most insectivorous bats catch their prey in flight, therefore flight style and wing morphology are very important in prey selection [38-40]. Agility and manoeuvrability of flight are for a great part defined by the wing loading, bodyweight-forearm ratio is an alternative parameter that closely represents the wing loading [40,41]. Species with a lower bodyweight-forearm ratio can be considered to be more agile, because they have less mass in comparison to the size of the wing [42]. A higher bodyweight-forearm ratio relates to harder and larger insects in diets. These insects are easier detected from larger distances and therefore agility is less important. Pipistrellus and Hipposideros species differed significantly in their bodyweight-forearm ratio, from which we can infer that Pipistrellus species are generally more agile allowing them to catch smaller prey such as Diptera. This is reflected in the diets of both genera; where Hipposideros species mainly feed on harder insects and Lepidoptera, the diet of Pipistrellus species often consists of softer species.
The large differences in skull morphology among bat species is an important factor in predicting and understanding the diets of bats [43,44]. Harder insects are more often consumed by bats that have robuster jaws and thus have a stronger bite force [11,12,45]. Bite force is also related to the thickness of the teeth enamels. Therefore bats with thicker enamels often feed on harder insects [46]. Species that lack these traits are therefore more likely to select softer prey. We used the longest cranial length to represent bite force. Our results show that cranial size and thus bite force is a better predictor than bodyweight for the diets containing soft and hard insects. This indicates that not just the size of the bat determines the diet but that certain morphological characteristics of the skull are much more important [11-13,44].
Lepidoptera are relatively large, and bats don't need to be very agile to catch these insect in comparison to smaller insects. To detect larger insects, a low frequency should normally suffice [17] should normally suffice to detect larger insects, however, high frequency calls are actually needed to feed efficiently on Lepidoptera. Several moth species are known to easily detect lower call frequencies and subsequently avoid the bats [16,47,48]. Other Lepidoptera species can even jam the echolocation call which makes it very difficult for the bat to detect the prey [16,49]. Call frequency was relative strongly related to Lepidoptera content, while cranial length and bodyweight did not show any relationship with the dietary content of Lepidoptera. With higher peak frequencies the amount of Lepidoptera increased and the amount of other insect reduced. Especially Rhinolophus and Hipposideros species fed most on Lepidoptera.
We have shown that bodyweight-forearm ratio and cranial morphology rather than echolocation are good predictors for the content of hard or soft insects in the diets of bats. Whereas echolocation is the one parameter that determines Lepidoptera content. These traits relate strongly to prey selectivity. While some morphological characteristics can used to quantified diets to some degree, other external variables are also important in determining the diets of insectivorous bats e.g. season, local insect community composition or geographic range [2,19,50,51]. These external factors make it difficult to only use dietary analysis for assessing food preferences in bats. Additional difficulties arise from large biases in assessing the food supply, and comparing this to the consumed food items [6]. We propose that a combination of dietary analysis and morphological analysis is needed to make stronger inference about prey preference.
Acknowledgements
The authors would like to thank the faculty of Agriculture, Natural Resources and Environment of Naresuan University and the Cat Drop Foundation for financial support of this study. We kindly thank Mr. William Newcomb for proofreading an early draft of this manuscript.
References
- Boyles, J. G., Cryan, P. M., Mccracken, G. F., Kunz, T. H. (2011) Economic importance of bats in agriculture. Science 332:41–42.
- Leelapaibul, W., Bumrungsri, S., Pattanawiboon, A. (2005) Diet of wrinkle-lipped free-tailed bat (Tadarida plicata Buchannan , 1800) in central Thailand: insectivorous bats potentially act as biological pest control agents. Acta Chiropterologica 7:111–119.
- Reiskind, M. H., Wund, M. A. (2009) Experimental assessment of the impacts of northern long-eared bats on ovipositing Culex (Diptera: Culicidae) mosquitoes. Journal of Medical Entomology 46:1037–1044.
- Rydell, J., McNeill, D. P., Eklof, J. (2002) Capture success of little brown bats (Myotis lucifugus) feeding on mosquitoes. Journal of Zoology 256:379–381.
- von Helversen, O, Winter, Y. (2003) Glossophagine bats and their flowers: costs and benefits for plants and pollinators. In “Bat Ecology” Ed by TH Kunz, MB Fenton, Chicago University Press, Chicago, pp 346-389
- Whitaker, J. O., Mccracken, G. F., Siemers, B. M. (2009) Food habits analysis of insectivorous bats. Pages 567–592 in T. H. Kunz and S. Parsons, editors. Ecological and Behavioral Methods for the Study of Bats. 2nd edition.
- Barclay, R. M. R., Brigham, R. M. (1991) Prey detection, dietary nich breadth and body size in bats: why are aerial insectivorous bats so small. American Naturalist 137:693–703.
- Ghazali, M., Dzeverin, I. (2013) Correlations between hardness of food and craniodental traits in nine Myotis species (chiroptera, vespertilionidae). Vestnik Zoologii 47:67–76.
- Bogdanowicz, W., Fenton, M. B., Daleszczyk, K. (1999) The relationships between echolocation calls, morphology and diet in insectivorous bats. Journal of Zoology 247:381–393.
- Nogueira, M. R., Peracchi, A. L., Monteiro, L. R. (2009) Morphological correlates of bite force and diet in the skull and mandible of phyllostomid bats. Functional Ecology 23:715–723.
- Aguirre, L. F., Herrel, A., Van Damme, R., Matthysen, E. (2003) The implications of food hardness for diet in bats. Functional Ecology 17:201–212.
- Freeman, P. W. (1981) Correspondence of food habits and morphology in insectivorous bats. Journal of Mammalogy 62:165–173.
- Cakenberghe, V. Van, Herrel, A., Aguirre, L. F. (2002) Evolutionary relationships between cranial shape and diet in bats (Mammalia: Chiroptera). Pages 205–236 in P. Aerts, K. D’Aout, A. Herrel, and R. Van Damme, editors. Topics in Functional and Ecological Vertebrate Morphology. Shaker Publishing.
- Stevens R. D. (2005) Functional morphology meets macroecology: size and shape distributions of New World bats, Evol. Ecol. Res. 7, 837–851.
- Fenton, M. B., Bogdanowicz, W. (2002) Relationships between external morphology and foraging behaviour: bats in the genus Myotis. Canadian Journal of Zoology 80:1004–1013.
- Jones, G. (2005) Echolocation. Current Biology 15:R484–R488.
- Houston R. D., Boonman A. M., Jones G. (2004) Do echolocation signal parameters restrict bats' choice of prey? In “Echolocation in Bats and Dolphins” Ed by J Thomas, C Moss, M Vater, Chicago University Press, Chicago, pp 339–345
- Jones G., Rydell J. (2003) Attack and defense: Interactions between echolocating bats and their insect prey. In “Bat Ecology” Ed by TH Kunz, MB Fenton, Chicago University Press, Chicago, pp 301–345
- Kurta, A., Whitaker, J. O. (1998) Diet of the endangered Indiana bat ( Myotis sodalis ) on the northern edge of its range. The American Midland Naturalist 140:280–286.
- Whitaker, J. O., Castor, L. (2010) Identification of insect parts found in bat guano. Pages 567–592 in T. H. Kunz and S. Parsons, editors. Ecological and Behavioral Methods for the Study of Bats. 2nd edition. Johns Hopkins University Press, Baltimore, US.
- Zuur, A. F., Ieno, E. N., Smith, G. M. (2007) Analysing Ecological Data. Page 685 (M. Gail, K. Krickeberg, J. Samet, A. Tsiatis, and W. Wong, Eds.). 1st edition. Springer, New York.
- Herberich, E., Sikorski, J., Hothorn, T. (2010) A robust procedure for comparing multiple means under heteroscedasticity in unbalanced designs. PloS one 5:e9788.
- RStudio. (2012) RStudio: Integrated development environment for R (Version 0n.96.331). Boston, MA.
- R Development Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Oksanen, J. (2011) Multivariate Analysis of Ecological Communities in R: VeganTutorial.
- Zeileis, A. (2004) Econometric computing with HC and HAC covariance matrix estimators. Journal of Statistical Software 11:1–17.
- Dunn, P. K., Smyth, G. K. (2001) Tweedie family densities: methods of evaluation. Pages 1–8 Proceedings of the 16th International Workshop on Statistical Modelling. Odense, Denmark.
- Kaas, R. (2000) Compound poisson distributions and GLM’s - Tweedie's distribution. Pages 3–12 COMPSTAT: Proceedings in Computational Statistics.
- Zuur, A. F., Ieno, E. N., Walker, N., Saveliev, A. A., Smith, G. M. (2009) Mixed Effects Models and Extensions in Ecology With R. Page 574. Springer New York, New York, NY.
- Dunn, P. K. (2013) Tweedie exponential family models.
- Evin, A., Baylac, M., Ruedi, M., Mucedda, M., Pons, J.-M. (2008) Taxonomy, skull diversity and evolution in a species complex of Myotis (Chiroptera: Vespertilionidae): a geometric morphometric appraisal. Biological Journal of the Linnean Society 95:529–538.
- Aguirre L. F., Herrel A., van Damme R., Matthysen E. (2002) Ecomorphological analysis of trophic niche partitioning in a tropical savannah bat community. Proceedings of the Royal Society B: Biological Sciences 269: 1271-1278
- Litsios, G., Pellissier, L., Forest, F., Lexer, C., Pearman, P. B., Zimmermann, N. E., Salamin, N. (2012) Trophic specialization influences the rate of environmental niche evolution in damselfishes (Pomacentridae). Proceedings of the Royal Society B: Biological Sciences 279:3662–3669.
- Ferry-Graham, L. a, Bolnick, D. I., Wainwright, P. C. (2002) Using functional morphology to examine the ecology and evolution of specialization. Integrative and Comparative Biology 42:265–277.
- Chase J. (2011) Ecological niche theory. In “The Theory of Ecology“ Ed by SM Scheiner, MR Willig, Chicago University Press, Chicago, pp 93-108
- Heller, K.G., van Helversen O. (1989) Resource partitioning of sonar frequency bands in rhinolophoid bats. Oecologia 80: 178–186
- Jones G., Rydell J. (2003) Attack and defense: Interactions between echolocating bats and their insect prey. In “Bat Ecology” Ed by TH Kunz, MB Fenton, Chicago University Press, Chicago, pp 301–345
- Dechmann, D. K. N., Safi, K., Vonhof, M. J. (2006) Matching morphology and diet in the disc-winged bat Thyroptera tricolor (Chiroptera). Journal of Mammalogy 87:1013–1019.
- Isaac, S. S., Arimuthu, G. Μ. (1997) Development of wing morphology in the Indian pygmy bat Pipistrellus mimus. Journal of Biosciences 22:193–202.
- Salsamendi, E., Aihartza, J., Goiti, U., Almenar, D., Garin, I. (2005) Echolocation calls and morphology in the Mehelyi’s (Rhinolophus Mehelyi) and Mediterranean (R. euryale) horseshoe bats: implications for resource partitioning. Hystrix 16:149–158.
- Šuba, J. U., Vintulis, V. I., PÃÆââ¬Å¾Ãâââ¬Återsons, G. U. (2011) Body weight provides insight inrto the feeding strategy of swarming bats. Hystrix 22:179–187.
- Elangovan V., Priya Y. S., Marimuthu G. (2011) Postnatal development, wing morphology and flight preformance of the short-nosed fruit bat, Cynopterus sphinx. In “Bats – Biology, Behavior and Conservation” Ed by JL Zupan, SL Mlakar, Nova Science Publishers, New York, pp 221-252
- Arita, H. T., Fenton, M. B. (1997) Flight and echolocation in the ecology and evolution of bats. Trends in Ecology & Evolution 12:53–8.
- Barlow, K. E., Jones, G., Barratt, E. M. (1997) Can skull morphology be used to predict ecological relationships between bat species? A test using two cryptic species of pipistrelle. Proceedings of the Royal Society B: Biological Sciences 264:1695–700.
- Freeman, P. W. (1998) Form, function, and evolution in skulls and teeth of bats. Papers in Natural Resources 9:140–156.
- Dumont, E. R. (1995) Enamel thickness and dietary adaptation among extant primates and chiropterans. Journal of Mammalogy 76:1127–1136.
- Miller, L. A., Surlykke, A. (2001) How some insects detect and avoid being eaten by bats: tactics and countertactics of prey and predator. BioScience 51:570.
- Schoeman, M. C., Jacobs, D. S. (2003) Support for the allotonic frequency hypothesis in an insectivorous bat community. Oecologia 134:154–62.
- Corcoran, A. J., Conner, W. E. (2012) Sonar jamming in the field: effectiveness and behavior of a unique prey defense. The Journal of Experimental Biology 215:4278–4287.
- Moosman, P. R., Thomas, H. H., Veilleux, J. P. (2012) Diet of the widespread insectivorous bats Eptesicus fuscus and Myotis lucifugus relative to climate and richness of bat communities. Journal of Mammalogy 93:491–496.
- Brian, M., Hickey, C., Acharya, L., Pennington, S. (1996) Resource partitioning by two species of vespertilionid bats (Lasiurus cinereus and Lasiurus borealis) feeding around street lights. Journal of Mammalogy 77:325–334.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences