Bioplastics and the Environment
Abolfazl Jafari-Sales, Ahmmad Reza Shahniani, Yashar Bagherizadeh, Sajad Alizadeh, Mehdi Jahangiri-Hoseinabadi, Mahboubeh Abdoli-Seuejani, Zahra Bamzadeh, Farnaz Rasi-Bonab
1Department of Microbiology, Kazeroon Branch, Islamic Azad University, Kazeroon, Iran
2Young Researchers and Elite Club, Ahar Branch, Islamic Azad University, Ahar, Iran
3Young Researchers and Elite Club, Tabriz Branch, Islamic Azad University, Tabriz, Iran
4Department of Microbiology, Shahre Kord Branch, Islamic Azad University, Shahre Kord, Iran
5Young Researchers and Elite Club, Marand Branch, Islamic Azad University, Marand, Iran
- Corresponding Author:
- Tel: +98(0) 9147611841
Fax: +98(0) 4142274746
E-mail: A.jafari_1392@yahoo.com
Received date: May 06, 2017; Accepted date: July 18, 2017; Published date: July 25, 2017
Citation: Jafari-Sales A, Shahniani AR, Bagherizadeh Y, et al. Bioplastics and the Environment. Electronic J Biol, 13:3
Abstract
The population growth in the present era and the great advances made in life has made the human beings inclined towards various industries and materials as a result of which the production and the accumulation of the waste materials in the environment are inevitable. Plastic industries are among the most important and most frequently applied industries and they are widely used and also the increase in the effects brought about by the non-degradable plastic waste has been turned to a growing concern. Polyhydroxyalkanoate (PHA) and Polyhydroxybutyrate (PHB), naturally produced by a great number of the microorganisms, can be considered as a substitute for the ordinary plastics and, unlike the plastics derived from petroleum, PHAs are completely biodegraded in one year by the microorganisms and turn to water and carbon dioxide and return to the nature. PHA and PHB are polyesters produced under imbalanced growth conditions in microorganisms. These polyesters are comprised of hydroxy fatty acids and they are considered as a relatively complex class of reservoir polymers synthesized by bacteria and archaea and deposit in cell cytoplasm in the form of nanometersize components.
Keywords
Bioplastic; Biodegradable; PHA; PHB.
1. Introduction
Plastics are used in manufacturing industries including automobiles to medicine. Plastics are very useful because, as synthetic polymers, their structure can be manipulated chemically and they can be given a wide spectrum of length and shape. Their molecular weight is in a range of 50000 to 1000000 Dalton (Da) [1]. The US annually discards about 25 million tones of home plastic wastes and these waste materials are usually disposed in garbage dumping landfills where they might remain undegraded for ten years [2]. Since plastics account for 20% of the urban solid wastes volume, there is a high incentive for the society to find alternative dumping methods [3]. The conventional plastics, used at present, are made of petroleum material, undegradable sources [4]. These materials are predominantly made through carboncarbon binds which resist degradation. Therefore, recycling plastics plays a significant role in conserving the environment. Plastics are readily recyclable. For example, polyethylene can be washed, melted and changed five times in their physical characteristics [5]. Polyvinyl chloride and poly acetone are used in manufacturing plastics to a great extent. Plastics can easily take any favorable form like fibers and thin films. They have a high chemical resistance and their elasticity might be very much or very little. Thus, they have found their ways into some disposable and reusable products as packing material. The difficulty with which such plastic products can be degraded or recycled is their unfavorable feature. The plastics, featuring a xenobiotic nature, are problematic in microbial degradation [6]. In the recent years, there has been a growing trend of public worries related to the harmful effects of plastics derived from petroleum material on the environment. The mechanisms devised by the nature and the self-regulating abilities cannot fight back the new pollutants because these mechanisms and devices used by nature are unfamiliar with plastic materials and this has made some countries begin developing biodegradable plastics.
According to the estimations, more than 100 million plastics are produced annually. The plastics annual use in the US is 80 kg per capita and it is 60 kg per capita in European countries and it is 2 kg per capita in India [7]. Forty percent of the 70 billion pound plastics produced annually are discarded in landfills. Several hundred tons of plastic are annually discarded to the marine environment and finally pile up in the oceanic regions. The solution to getting rid of the undegradable plastics is burning them but, besides being cost-intensive, such a method is dangerous because harmful chemical compounds such as hydrogen chloride and hydrogen cyanide are released in burning processes [8]. There are also substantial flaws in recycling plastic materials because classification of many types of plastics is problematic and also there are changes made in plastic materials that have rendered their usability cover a wide spectrum. Replacing the degradable plastics with non-biodegradable plastics benefits the plastic industry and the decision-makers. Manufacturing environment-friendly products like bio- plastics helps us overcome the form of contamination created by undegradable plastics [9].
2. A Solution for the Plastics
Three types of biodegradable plastics, namely photodegradable, semi-biodegradable and perfectly biodegradable plastics, are currently being used. Photodegradable plastics have light-sensitive groups which are directly inserted in their polymeric structure as additives. High rate of UV radiation can degrade their polymeric structure and make them prone to further degradation by bacteria but because the dumping areas do not receive enough of the sunlight, they, consequently, remain undegraded [7]. Semi-degradable plastics are starchy plastics in which starch is inserted into their composition for holding their short polyethylene parts. When the starchy plastics are discarded in the landfills, the bacteria living in the soil attack the starch and free the polymeric parts that can, resultantly, be degraded by the other bacteria. In fact, the bacteria attack the starch and convert it to polyethylene parts and therefore they are rendered degradable. The third type includes the biodegradable plastics which are relatively new and promising and these are used by bacteria as biopolymer among which the followings can be pointed out:
Polyhydroxyalkanoate (PHA), Polylactive (PLA), Aliphatic Polyesters, polysaccharides, copolymers and a combination of the aforesaid cases [9].
2.1 Polyhydrozyalkanotes
PHA is a family of bio-polyesters featuring diverse structures and it is the only bio-plastic that can be synthesized by microbes. PHA can be synthesized by more than 30% of the soil-borne bacteria [10]. These polymers accumulate intracellular for about 90% of the cell’s dry weight under nutritional stress conditions and they are served as the carbon resources and energy storage [1]. Some bacteria are active in the sludge in the sea floor regions and in extreme environments and they also have the ability to make PHA. During the past decade, PHA has been developed to serve various uses [11]. PHA’s molecular mass is about 50000 to 1000000 Dalton which is different from the same of the PHA producers. Monomeric units, as well, exist in D configuration as a result of the spatial features belonging to biosynthetic enzymes [12-15]. PHA has structure-related rich features. PHA’s homopolymers, random copolymers and blocking copolymers can be produced depending on the type of the bacteria and the growth conditions. More than 150 monomers have been reported for PHAs. PHAs have changeable mechanical and thermal features as well as use diversities such as in environment-favorable biodegradable plastics which are currently used for packing, fibers, degradable and biocompatible implants. They also facilitate drug releasing transporters and PHA monomers can also be used for the development of bio-fuels, drugs or chiral intermediates. PHA oligomers have also been reported as nutrients for animals. Due to these advances, microbial PHA composes a valuable chain of industrial fermentation, materials, drugs and biofuels to the excellent chemical compounds. More than 20 companies have been established worldwide to commercialize such progresses [16,17].
2.2 PHA biosynthesis
PHA can be synthesized by means of chemical substances or by taking advantage of biological methods. PHA biosynthesis leads to the generation of compounds having high molecular weight in contrast to the chemical methods applied. Therefore, PHA biosynthesis allows for a greater control over the monomeric structures in PHA polymers. PHA polymerase (PHA’s synthase)’s features influence the monomers combined in polymers. Thus, PHA synthesis is carried out by grown microorganisms in an aquatic solution containing stable resources such as starch, glucose, sucrose, fatty acids and even the nutrients extant in sewage below a temperature ranging from 30°C to 37°C [11].
2.3 Prokaryotic PHAs
The greatest number of PHAs is produced by prokaryotic groups such as bacteria and archaeas, though transgenic plants have also been reported for the production of PHA. Prokaryotic PHA functions as energy and carbon storage as well as increasing the survival under various environmental conditions therefore all PHA-related applications are prokaryotic [17-19].
2.4 PHA biosynthesis in natural isolates
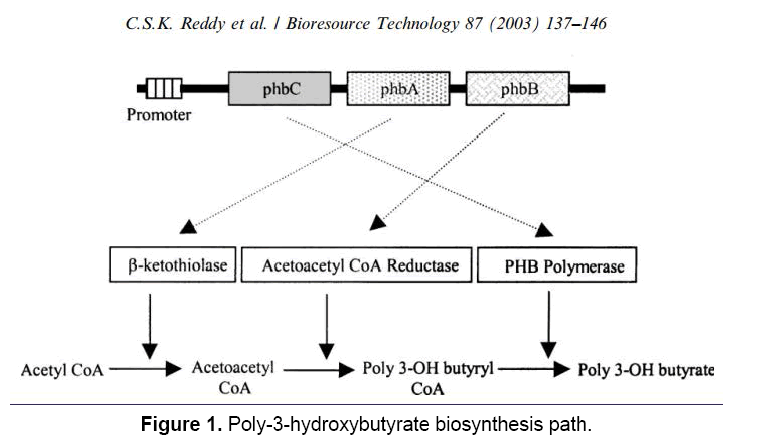
There are extensive information provided regarding the metabolism, biochemistry and physiology of poly- 3-hydroxybutyrate up to 1987 through the relevant molecular genetics studies. Numerous coding genes involved in the formation and degradation of cloned PHA and from various types of microorganisms have been identified and the genetic research provide us with the regulation and formation of PHA based on different growth conditions. The primary roles of central metabolism and cell physiology have been revealed through studies on PHA mutants via genetic manipulation of PHB genes [1]. Such studies provide specifications regarding microbial physiology and they are considered as powerful tools for the design and engineering of recombinant organisms for the production of PHA. Biosynthesis path of poly-3- hydroxy butyrate includes three enzymatic reactions catalyzed by three different enzymes.
The first reaction includes two molecules of acetyl coenzyme A turned to Acetyl CoA by beta ketoacyl- CoA thiolase (encoded by PHB A). The second reaction involves acetoacetyl-CoA’s reduction to 3-hydroxybutyrile-CoA which is polymerized to PHB by poly-3-hydroxybutyrate polymerase (encoded by PHB C) (Figure 1) [1].
2.5 PHA production by recombinant bacteria
Producing natural PHA by bacteria takes a long time and it needs optimum production temperatures. It is difficult to lyse these bacteria and they follow certain processes to degenerate PHA. Bacteria such as E. coli are unable in synthesizing or degenerating PHA. Therefore, E. coli grows fast in high temperature and it can be easily lysed. Fast growth makes the bacteria capable of accumulating large amounts of polymer. Cell lysis allows for cost-effective PHA granules purification [1,20,21]. Metabolism engineering for the purpose of launching new metabolic paths for expanding the range of the usable substrate has been intensively investigated so as to elevate PHA synthesis and produce new PHAs. Recombinant E. coli strains insert in them the PHA biosynthesis genes from Alcoligenes eutrophas in a stable plasmid featuring a high number of copies and they are applied to enhance PHA productivity [21,22]. Thus, E. coli can take advantage of variegated carbon sources such as glucose, sucrose, xylose and similar substrates like molasse and hemicelluloses can be applied to reduce the costs when working with PHA. Such a strategy can be generalized to almost all bacteria if the given bacterium is found having more metabolic symbionts than what is currently consumed [23]. The heterologous expression of PHA biosynthesizing gene of the bacterium A. eutrophic in Psedomunas oleavorans facilitates a mixture of poly- 3-hydroxybutyrates and Msc-PHA [24]. There are two different approaches adopted in developing the bacterial strains producing PHA from cheap carbon substrates. Firstly, substrate-applying genes can be introduced to the PHA producers. Secondly, PHA biosynthesizing genes can be introduced to non-PHA producers making use of cheap substrate. For now, the second approach seems more promising [25].
2.6 PHA featuring biodegradability
The feature distinguishing PHA from plastics produced of petroleum material is the biodegradability of PHA. PHAs degrade through being exposed to the soil, composts or marine sediments. Biodegradability depends on some factors such as bacterial activity in the environment and being exposed to surface, moisture, temperature, pH and molecular weight [26]. Polymeric composition and crystallization are also considered as important factors for PHA. It has also been verified that the nature of monemeric units influence the degradation [27]. There are found copolymers containing PHB monomeric units that can be degraded to 3-hydroxybutyrate-co-3- hydroxyvalerate (3-HB-Co-3HV) more quickly.
Microorganisms secrete enzymes that break polymers to their molecular constituents, hydorxyacids, used as carbon sources for growth. PHA biodegradation under aerobic conditions leads to the production of CO2 and H2O, whereas, the carbon hydrolysis products are dioxide and methane under anaerobic conditions [6,8]. PHAs can be turned to composts in a wide spectrum of temperatures, even in maximum 60°C with a moisture rate of 55%. Studies show that 85% of PHAs can be degraded within seven weeks. PHAs can also be degraded in aquatic environments within 254 days even in disallowed temperatures, 6°C [8].
2.7 PHA uses
PHAs as packing material: PHAs are primarily used in materials used by us on a daily basis such as shampoo bottles and packaging materials. PHAs are also of use in packing films which are predominantly developed for use as freezer bags, paper covers, containers, disposable items like shavers, kitchen and medical containers, feminine hygiene products, utensils, cosmetic tools and medical and surgical uniforms, home furniture and instruments, carpet, packing, fertilizer bags and so forth [28,29].
PHA as medical implant materials: During the past 200 years, PHA and its derivatives have been applied for the development of tools such as stitches, stitch sewers, coatings, clasps or crampons, screws, epiphyseal plate systems and bone plating, tumor surgeries, heart stents, tendon repairing tools, devices used in repairing the inter-ventricular wall deformities, bulking agents, venous valves, bone marrow scaffolds.
It has been shown in another study that 3HB boosts the cell propagation in cultured L929 cells as well as in high cellular densities. Although 3HB has not been evidenced influencing the cellular cycles it has been found considerably curbing the cellular death.
Cheng et al found out that PHBHHX microparticles enhances the propagation of rats’ fibroblast L929 cell and it causes an incerase in intacellular calcium concentration.
Oligo-3-hydroxybutyrate or OHBs are found in various organisms. They can constitute complexes with inorganic polyphosphates, nucleoic acids and proteins [30-32].
PHA as drug delivery transporters: Lactate and glycolate homopolymers and copolymers are widely used commercially as releasing products for drug delivery. Therefore, lactate and glycolate copolymers are predominantly degenerated via hydrolysis. Thus, drug release cannot be completely controlled. In early 1990, PHA was considered as a candidate drug transporter due to its ready biodegradability, biocompatibility and degradation resulting from surface erosion. Among various PHAs, only PHB and PHBV are applied as controlled drug delivery agents [30-33].
PHA as bio-fuels: Recently, Zhang et al. [22] showed that acetomethyle 3-hydroxybutyrate (3HBME) and acetometyle 3-hydroxyalkanoate (3HAME) obtained from PHB and PHA featuring a medium chain length can be used as a biofuel. They investigated differential groups, namely 3HBME, 3HAME, ethanol, n-propanol, n-butanol, gasoline and mixed 3HAME- and 3HMBE-based fuels and found out that 3HBME and 3HAME possess 30 and 30 Kj, respectively.
PHA monomers as drugs: 3-D-hydroxybutyrate, 3-DL-hydroxybutyrate and 3HBME salts are derivatives of 3HB. D-3HB is the most common product of microbial PHA degeneration used for tissue engineering applications. 3HB and its derivatives exert effects on cellular apoptosis and rats’ cytosolic glial cell Ca2+ concentration and they have been found causing a reduction in apoptosis and an increase in the cytosolic cell’s Ca2+ concentration. The effects of 3HB derivatives on cytosolic Ca2+ concentration can be reduced by Nitrepidin [34].
2.8 Polyhydroxybutyrate
PHB was first discovered in 1925 by Lemoigene who described it a lipidic inclusion in megaterium bacillus bacteria [35]. Later, it was figured out that PHB is a polymer featuring high mollecular weight involved in carbon and energy storage by a certain types of microorganisms. Because the bacteria synthesizes and degrade PHB, it is economically and environmentally an alternative method for petroleum- based plastics for the production of which degradable resources are consumed and waste materials are produced. From thousnads of polymers, PHAs have globally undergone the highest number of developmental and research efforts during the past ten years because their physical characteristics are comparable with the conventional plastics. PHA, a polyhydroxybutyrate-co-valerate (PHVB), was previously produced under the commercial name of Biopol [36].
The more fascinating feature of PHB and the other PHAs, which is also considered as one of their advantages, is that they perfectly degrade aerobically to Co2 and H2O [37,38]. PHB possesses better oxygen-tight characteristics in respect to polypropylene and polyethylene tetraphetalate. It also exhibits better water vapor insulation characteristics in respect to polypropylene and it also has anti-odor and anti-lipid peroxidation features rendering it appropriate for food studd packaging [39]. Moreover, this polymer has a better resistance to UV radiations in comparison to polypropylene and it displays thermal resistance as high as 130°C. PHB is completely nontoxic in mamals because it possesses a LD50 more than 5000 mg/kg and, in fact, it is applied in denitrification of the pot water [39,40]. Considering all the aforementioned features, PHB efficiency is restricted to its low solidity of the effects it poses. Its flexibility to breaking is 6% as compared to a value of 40% scored for polypropylene [41,42]. Another disadvantage of PHB is that it degrades almost ten degrees above its boiling point, 177°C and this is problematic in processing [37]. The solution lies in appending long-chain units of hydroxy acid monomers such as hydroxy valerate to the PHB structure which makes the polymere more solid and more flexible [39]. Polyhydroxybutyrate-co-valerate (PHBV) was discovered in 1983 and PHB copolymere has been extensively studied up to the present time. PHBV copolymer contains 25% valerate and it is more flexible in comparison to PHB and it indicates five time as much reduction in Young’s Modulus in comparison to a 7% GPa [39]. Young’s modulus is defined as stress to strain ratio.
Hydroxy Valerate was added [42]. Also, the melting point is reduced to some extent without exerting any efect on the degradation temperature and makes it an easier copolymer for the process [43,44].
3. Conclusion
Recent advances in metabolic engineering are supported by the genomic information and bioinformatics that have allowed for an array of opportunities for initiating new metabolic paths. This helps us not only in expanding the domain of the substrate usable in manufacturing PHA but it also increases the current PHA output. The studies should also be concentrated on understanding the host-plasmid interactions as a result of which more stable plasmids can be created and it is also worth mentioning that the recombinant bacteria are suuperior to the wild-type bacteria in terms of their consumption for producing PHAs. Transgenic plants that carry PHA biosynthesizing microbial genes have lately been developed aiming at decreasing the total price of polymer production. Therefore, there is a need for further research in this area for increasing the bioplastics manufacturing as successful alternatives for the undegradable plastics.
References
- Madison LL, Huisman GW. (1999). Metabolicengineering of poly (3-hydroxyalkanoates): From DNA to plastic. Microbiol Mol Biol Rev. 63: 21-53.
- Palmisano AC, Pettigrew CA. (1992). Biodegradability of plastics. BioScience. 42: 968-685.
- Stein RS. (1995). Polymer recycling: Thermodynamics and economics. In: Rader CP, Baldwin SD, Cornell DD, Sadler GD, Stockel RF (eds) Plastics, Rubber, and Paper Recycling. American Chemical Society, Washington, DC.
- Pavia DL, Lampman GM, Kriz GS. (1988). Introduction to Organic Laboratory Techniques, 3rd Ed. Saunders, Fort Worth, TX.
- Rader CP, Stockel RF. (1995). Polymer recycling: an overview. In: Rader CP, Baldwin SD Cornell DD, Sadler GD, Stockel RF (eds) Plastics, Rubber, and Paper Recycling. American Chemical Society, Washington, DC.
- Flechter A. (1993). In: Plastics from Bacteria and for Bacteria: PHA as Natural, Biodegradable Polyesters. Springer Verlag, New York. 77-93.
- Kalia VC, Raizada N, Sonakya V. (2000). Bioplastics. J Sci Ind Res. 59: 433-445.
- Johnstone B. (1990). A throw away answer. Far Eastern Econ Rev. 147: 62-63.
- Song SS, Hein S, Steinbuchel A. (1999). Production of poly (4-hydroxybutyric acid) by fed-batch cultures of recombinant strains of Escherichia coli. Biotechnol Lett. 21: 193-197.
- Wu Q, Sun SQ, Yu PHF, et al. (2000). Environmental dependence of microbial synthesis of polyhydroxyalkanoates. Acta Polym Sin. 6: 751-756.
- Chen GQ. (2009a). A polyhydroxyalkanoates based bio- and materials industry. Chem Soc Rev. 38: 2434-2446.
- Senior PJ, Beech GA, Ritchie GAF, et al. (1972). The role of oxygen limitation in the formation of poly-b-hydroxybutyrate during batch and continuous culture of Azobacter beijerinckii. Biochem J. 128: 1193-1201.
- Dawes EA, Senior PJ. (1973). The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 10: 135-266.
- Oeding V, Schlegel HG. (1973). Beta-ketothiolase from Hydrogenomonas eutropha HI6 and its significance in the regulation of polybeta-hydroxybutyrate metabolism. Biochem J. 134: 239-248.
- Wang JG, Bakken LR. (1998). Screening of soil bacteria for polybeta-hydroxybutyric acid production and its role in the survival of starvation. Microb Ecol. 35: 94-101.
- He WN, Zhang ZM, Hu P, et al. (1999). Microbial synthesis and characterization of polyhydroxyalkanoates by strain DG 17 from glucose. Acta Polym Sin. 6: 709-714.
- Castro-Sowinski S, Burdman S, Matan O, et al. (2009). Natural functions of bacterial polyhydroxyalkanoates. Microbiol Monogr. 3: 31-61.
- Reusch RN. (1987). Poly-beta-hydroxybutyrate calcium polyphosphate complexes in eukaryotic membranes. Proc Soc Exp Biol Med. 191: 377-381.
- Steinb€uchel A, Schlegel HG. (1991). Physiology and molecular genetics of poly(b-hydroxy-alkanoicac id) synthesis in Alcaligenes eutrophus. Mol Microbiol. 5: 535-542.
- Wang JG, Bakken LR. (1998). Screening of soil bacteria for polybeta-hydroxybutyric acid production and its role in the survival of starvation. Microb Ecol. 35: 94-101.
- Zhang H, Obias V, Gonyer K, et al. (1994). Production of polyhydroxyalkanoates in sucrose-utilizing recombinant Escherichia coli and Klebsiella strains. Appl Environ Microbiol. 60: 1198-1205.
- Lee SY, Yim KS, Chang HN, et al. (1994). Construction of plasmids, estimation of plasmid stability and use of stable plasmids for the production of poly (3-hydroxybutyric) acid by 24 recombinant Escherichia coli. J Biotechnol. 32. 203-211.
- Lee EY, Jendrossek D, Schirmer A, et al. (1995). Biosynthesis of copolyester consisting of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids from 1,3-butanediol or from 3-hydroxybutyrate by Pseudomonas sp. A33. Appl Microbiol Biotechnol. 42: 901-909.
- Preusting H, Kingma J, Huisman G, et al. (1993). Formation of polyester blends by a recombinant strain of Pseudomonas oleovorans: Different poly (3-hydroxyalkanoates) are stored in separate granules. J Environ Polym Degrad. 1: 11-21.
- Lee SY. (1996a). Bacterial polyhydroxyalkanoates. Biotechnol Bioeng. 49: 1-14.
- Boopathy R. (2000). Factor limiting bioremediation technologies. Bioresour Technol. 74: 63-67.
- Lee SY. (1996b). Plasticbac teria Progress and prospects for polyhydroxyalkanoates production in bacteria. TIBTECH. 14: 431-438.
- Mikova G, Chodak I. (2006). Properties and modification of poly(3-hydroxybutanoate). Chem Listy. 100: 1075-1083.
- Clarinval AM, Halleux J. (2005). Classification of biodegradable polymers. In: Smith R (ed) Biodegradable polymers for industrial applications. CRC, Boca Raton. 3-56.
- Wang ZH, Wu HN, Chen J, et al. (2008b). A novel self-cleaving phasin tag for purification of recombinant proteins based on hydrophobic nanoparticles. Lab Chip. 8: 1957-1962.
- Bian YZ, Wang Y, Guli S, et al. (2009) Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials. 30: 217-225.
- Chen GQ, Wu Q. (2005). Polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 26: 65-6578.
- Yao YC, Zhan XY, Zou XH, et al. (2008). A specific drug targeting system based on polyhydroxyalkanoate granule binding protein PhaP fused wit targeted cell ligands. Biomaterials. 29: 4823-4483.
- Xiao XQ, Zhao Y, Chen GQ. (2007). The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials. 28: 3608-3616.
- Gilmore DF, Fuller RC, Lenz R. (1990). Biodegradation of poly (beta-hydroxyalkanoates). In: Barenberg SA, Brash JL, Narayan R, Redpath AE (eds) Degradable Materials: Perspectives, Issues and Opportunities. CRC Press, Boca Raton, FL.
- Luzier WD. (1992). Materials derived from biomass biodegradable materials. Proc Natl Acad Sci U S A. 89:839-842.
- Scott G. (1990). Photo-biodegradation of plastics. In: Barenberg SA, Brash JL, Narayan R, Redpath AE (eds) Degradable Materials: Perspectives, Issues and Opportunities. CRC Press, Boca Raton, FL.
- Williams MD, Rahn JA, Sherman DH. (1996). Production of a polyhydroxyalkanoate biopolymer in insect cells with a modified eukaryotic fatty acid sythase. Appl Environ Microbiol. 62: 2546-254.
- Galvin TJ. (1990). PHBV biodegradable polyester. In: Barenberg SA, Brash JL, Narayan R, Redpath AE (eds) Degradable Materials: Perspectives, Issues and Opportunities. CRC Press, Boca Raton, FL.
- Lee SY, Chang HN, Chang YK. (1994). Production of poly (P-hydroxybutyric acid) by recombinant Escherichia coli. Ann NY Acad Sci. 43: 53-721.
- Evans JD, Sikdar SK. (1990). Biodegradable plastics: an idea whose time has come? Chemtech. 42: 20-38.
- Tipler PA. (1991). Physics for scientists and engineers: Mechanics, oscillations and waves. Thermodynamics 5th Edn. 1: 1-650.
- Williams SF, Peoples OP (1996) Biodegradable plastics from plants. Chemtech. 38: 44-38.
- Lee SY, Chang HN. (1996). Characteristics of poly (3-hydroxybutyric acid) synthesis by recombinant Escherichia coli. Ann N Y Acad Sci. 133: 142-782.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences