Biological Properties of Iranian Zataria Multiflora Essential Oils: A Comparative Approach
Salome Dini, Abolfazl Dadkhah, Faezeh Fatemi
Salome Dini1,Abolfazl Dadkhah2*,Faezeh Fatemi3
1Young Researchers and Elite Club,Karaj Branch,Islamic Azad University,Karaj,Iran
2Faculty of Medicine,Qom Branch,Islamic Azad University,Qom,I.R. Iran
3Nuclear Fuel Cycle Research School,Nuclear Science and Technology Research Institute,Tehran,I.R. Iran
Received date: August 06,2015; Accepted date: September 16,2015; Published date: September 19,2015
Abstract
Zataria multiflora Boiss, known as Avishan-e- Shirazi in Persian, with traditional pharmacological properties take advantage of being useful medicinal plant. In this study, the chemical composition of different parts of Iranian Z. multiflora Boiss oils and also its antioxidant and antibacterial properties have been considered. The results showed that the main oil components especially thymol and carvacrol are identical in the oils exctracted derived from leaf, flower and aerial parts of the plant. In addition, the different part oils indicated the high radical scavenging and antioxidant properties. Also, E. coli and P. aeroginosa are more sensitive to the effects of the oil samples. However, the flower and aerial part oils of Z. multiflora are more efficient than the leaf in sensitivity to these microorganisms. These findings suggest the antioxidant and antibacterial activities of the oils derived from the leaf, flower and aerial parts of Z. multiflora regarding thymol and carvacrol as the major components. With a view to these biological properties, the applications of the oils in medicinal purposes are promising findings.
Keywords
Zataria multiflora; Essential oils; Antioxidant activity; Antibacterial activity.
Introduction
Zataria multiflora Boiss (Lamiaceae; Voucher Number: 41754) known as Avishan-e-Shirazi in Persian,is a thyme-like plant that grows wild in central and southern parts of Iran. It is an important flowering plant in Iranian traditional medicine. Several pharmacological properties such as antimicrobial,antifungal,anti-seizure,anti-nociceptive,anticandida,anti-septic,anti aphtous,analgesic,carminative and anti-inflammatory effects have been reported for this plant. Flavonoids and essential oils are the most important componentswith reported pharmacological activities [1-10]. It has been reported that the Z. multiflora essential oils can stimulate innate immunity [11] and have antibacterial,antifungal and antioxidant activities [12-15]. Moreover,the oil of Z. multiflora successfully inhibited the growth of bacteria associated with gastrointestinal infections,including Staphylococcus aureus,enterohemorrhagic Escherichia coli,Salmonella Typhi and Paratyphi,and Shigella flexneri and Bacillus cereus [16-18]. Its essential oil has also been proposed for disorders of respiratory and gastrointestinal system [19]. Moreover,the plant oil is a down-regulator of MDM2 gene expression which highlights the effectiveness of this oil in malignant disease [20].
To consider partly,the extensive uses of Z. multiflora in healthcare and medicine,in this study we investigated –for the first time- the chemical composition of different parts (i.e.,leaf,flower and aerial parts) of Iranian Z. multiflora Boiss oil extracts. Moreover,the antioxidant and antibacterial properties of these oils extracted from three divisions have been compared.
Materials and Method
Plant materials
Different members of fresh Z. multiflora i.e. flower,leaf and aerial parts (leaf,flower,and stem) were collected separately in May 2010 from the Shiraz city,Iran. Dr. Younes Asri (Botanist) from herbarium of Iranian botanical garden (TARI) authenticated the plant materials with the voucher number 41754.
Oil extraction and analysis
Oil extraction from the different Z. multiflora portions (air-dried) was carried out by hydrodistillation using a Clevenger-type apparatus [21]. The extractions were carried out for 2 h and the oils were stored in dark glass bottles in a freezer until further use. GC analysis was performed using a GC (9-A Shimadzu,Japan) equipped with a flame ionization detector (FID). Quantitation was carried out on Euro Chrom 2000 software (KNAUER Company) by the area normalization method. The analysis was carried out using a DB-5 fused-silica column (30 m × 0.25 mm,film thickness 0.25 μm) and a temperature program of 40-250°C at a rate of 4°C/min,injector temperature of 250°C,detector temperature of 265°C and the carrier gas was helium (99.99%). The GC/MS unit consisted of a Varian-3400 GC coupled to a Saturn II ion trap detector. The column of GC/MS was the same as of the GC under the same conditions that the above analysis was carried out. The constituents were identified by comparing their mass spectra with those in the computer library and with the authentic standards.
Radical-scavenging capacity (DPPH assay) of the oils
The hydrogen atom or electron donation abilities of the extracts and pure compounds were measured from the bleaching of the purple-colored methanol solution of 2,2-diphenylpicrylhydrazyl (DPPH). This spectro-photometric assay uses the stable radical DPPH as reagent [22,23]. Among the different concentrations of essential oil preparations,20% v/v (in methanol) was found suitable for DPPH test. Fifty μL of the essential oils in methanol was added to 5 ml of DPPH solution (0.004% DPPH in methanol). Trolox (1 mM),a stable antioxidant,was used as a synthetic reference. After 30 min of incubation period at room temperature,the absorbance was read against the blank at 517 nm. The inhibitory effects of the extracts in percent (I %) was calculated by the following formula:
I% = (Ablank – Asample/Ablank) ×100. Where,Ablank is the absorbance of the control reagent (containing all reagents except the test compound),and Asample is the absorbance of the test compound. All the assays were carried out in triplicate (22,23).
Β-Carotene-linoleic acid assay of the oils
The antioxidant activity of essential oils was determined using the ß-carotene bleaching test [24]. Approximately 10 mg of ß-carotene (type I synthetic) was dissolved in 10 ml of chloroform,and then 0.2 ml of this solution was added to a boiling flask containing 20 mg linoleic acid and 200 mg Tween 40. Chloroform was removed using a rotary evaporator at 40°C for 10 min. Then,50 ml of distilled water saturated with oxygen was added slowly with vigorous agitation to form an emulsion. The emulsion (5 ml) was added to a tube containing 0.2 ml of essential oil solution prepared according to [25]. The absorbance was immediately measured at 470 nm against a blank consisting of an emulsion without ß-carotene. The tubes were placed in a water bath at 50°C and the oxidation of the emulsion was monitored spectrophotometrically by measuring absorbance at 470 nm over a 60 min period. Samples containing 0.2 ml of ethanol instead of essential oils were also monitored and used as control. Butylated hydroxytoluene (BHT 1mM in ethanol),a stable antioxidant was used as reference. The antioxidant activity was expressed as inhibition percentage with reference to the control sample after 60 min of incubation,using the following equation:
AA =100(DRC - DRS)/DRC,where
AA = antioxidant activity %
DRC = degradation rate of control = [ln (a/b)/60]
DRS = degradation rate in presence of sample = [ln (a/b)/60]
a = absorbance at time 0
b = absorbance at 60 min
Determination of Minimal Inhibitory Concentration and Minimal Bactericidal Concentration
The minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) were assessed according to Baron et al.’s (1990) [26] procedure and NCCLS M2-A7 (2000) [27]. All tests were performed in BHB. MIC,defined as the lowest concentration of the oil resulted in a complete inhibition of visible growth in the broth,was determined as follows: A bacterial suspension was made in BHB to a density of 0.5 McFarland standards and confirmed by spectrophotometrical reading at 600 nm. A standardized suspension (100 μL) of the each test organisms,separately,was inoculated in to a series of sterile tubes of BHB containing twofold dilutions of cumin essential oils. The initial concentration of cumin essential oil in the first tube containing BHB was 1/2. This was used to prepare serial doubling dilutions over the range 25 to 0.048 mg/ml. A tube without essential oils was chosen as control. All tubes were incubated at 37°C for 24 h. Thereafter,the tubes were checked for growth of bacteria and turbidity from control tube to higher concentration [28,29]. To determine the MBC of oils,all tubes without bacterial growth were cultured on BHI and incubated at 37°C for 24h. The plates were checked for growth of bacterial colonies. MBC was evaluated as the lowest oil concentration at which no growth was observed on the plates.
Statistical analysis
Data are presented as means ± Standard Error (SE). The results were subjected to one-way ANOVA followed by Tukey’s HSD (Honestly Significant Differences) using SPSS13.0 software. The significance was determined as P<0.05.
Results
The chemical compositions of different parts of Z. multiflora essential oils
The oil extraction yields from the leaf,flower and aerial parts were ~ 1.07,2.5 and 3 % (w/w),respectively. GC/GC-MS analysis of the essential oils derived from three samples resulted in identification of 12 known compounds (Table 1). As shown,the main components of three parts including Leaf,flower and aerial parts were carvacrol and thymol. In addition,the compositions of the essential oil of different parts of Z. multiflora components by chemical classes are demonstrated in Table 2. As shown,the total oxygenated and phenolic compounds were identified as the major classes of the chemical compounds of essential oils in all parts (Table 2).
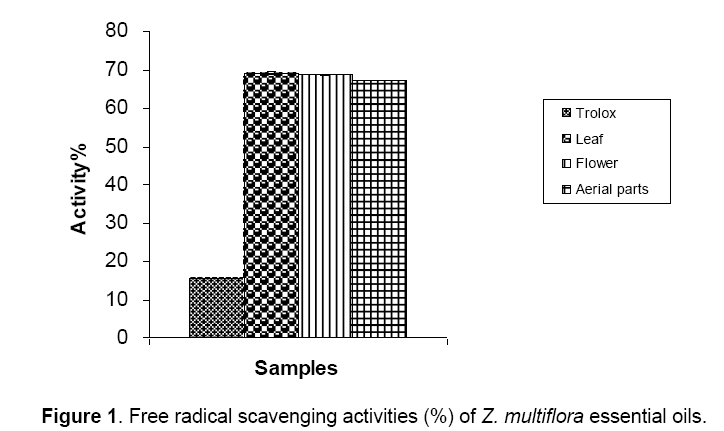
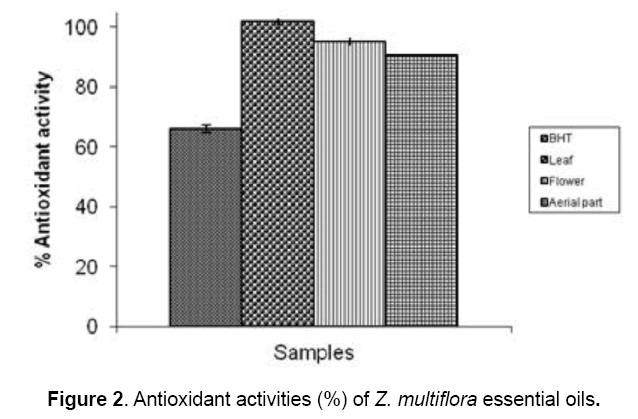
The antioxidant activities of different parts of Z. multiflora essential oils
The antioxidant properties of the different parts of Z. multiflora essential oils analyzed by DPPH and ß-carotene bleaching tests are presented in Figure 1&2. When compared to a standard antioxidant agent i.e. trolox (15%),it was found that the essential oils extracted from different parts of Z. multiflora had strong radical scavenging activity (~68%). Addition of the plant oils to the reaction mixture containing ß-carotene and linoleic acid,brought about ~95% inhibition in formation of peroxidation products that was equal in all plant parts (Fig. 2).
The radical scavenging activity of the oil samples were measured spectrophotometrically using DPPH free radical. Trolox was used as positive control. Results are mean ± SEM of three analyses carried out on essential oils derived from fresh and irradiated Z. multiflora Boiss. The antioxidant activity of the Z. multiflora oil samples were measured spectrophotometrically using ß-carotene bleaching test. BHT was used as positive control. Results are mean ± SEM of three analyses carried out on essential oils derived from fresh and irradiated Z. multiflora Boiss.
The antimicrobial activities of different parts of Z. multiflora essential oils
The results of MIC and MBC methods showed that E. coli as well as P. aeroginosa as Gram-negative bacteria (P>0.05),is more sensitive to the effects of the oils derived from leaf,flower and aerial parts as compared to B. cereus and S. aureus as Grampositive bacteria (P<0.05) (Table 3).
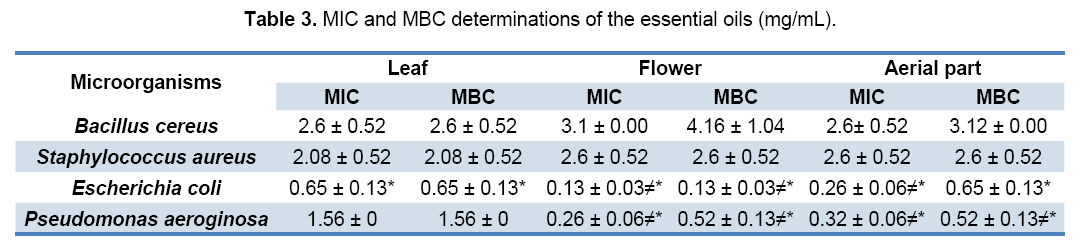
Data are mean ± S.E.M. of three different experiments. (*) denote significantly more sensitive from the nonemarked groups in each column (P<0.05). (≠) denote significantly more sensitive from the same group in the leaf (P<0.05).
Discussion
Our results revealed that despite the minor changing in the individual oil constitutes,the main components are the same in different parts of the plant. The major compositions containing carvacrol and thymol in the oils from three parts (leaf,flower and aerial parts) of Z. multiflora (Table,1) were in similarity with other studies [2,3,12,30-32]. Indeed,the oils isolated from different parts of Z. multiflora were found to be rich in oxygenated and phenolic compounds (Table. 2). These results are agreement with previous studies that reported the most abundant in Z. multiflora essential oils as oxygenated monoterpenes (approximately 70%) [4,33]. Gupta et al. reported that the essential oil of the Indian ecotype consists of 69% phenols containing mainly carvacrol [34].
Our data indicated that the essential oils derived from different parts of Z. multiflora i.e. leaf,flower and aerial parts had the equal radical scavenging and antioxidant properties of the different parts of the oils (Figs. 1&2). Other studies confirmed these results indicated that the essential oil of Z. multiflora possessed antioxidant activities as measured by the DPPH free radical scavenging and betacarotene bleaching assays [2,4,35]. Another study also showed the inhibitory effects of carvacrol and thymol derived from Z. multiflora aerial parts on nitric oxide and hydrogen peroxide production in glucosestimulated human monocyte [32].
The MIC and MBC of the oils derived from leaf,flower and aerial parts of Z. multiflora ranged from 0.5 to 4 mg/mL,indicating the sensitivity of all microorganisms e.g. E. coli,P. aeroginosa,B. cereus and S. aureus to the effects of the oils in different degree (Table 3). These data are in agreement with other studies indicating that the essential oils derived from aerial parts of Z. multiflora entirely inhibited the growth of yeasts at concentrations of less than 1 μL/mL. Moreover,the oils exhibited significant bacteriostatic and bactericidal activities against Gram-positive and Gram-negative bacteria at concentrations ranging from 0.12 to 8 μL/Ml. [36] The anti-microbial activity of most essential oils is related to their phenolic monoterpenes (4). Z. multiflora essential oil,with a very high percentage of thymol and carvacrol,possess significant anti-microbial activity [37]. Electron micrographs showed that these compounds,which are lipophilic in nature,do something on the cell membrane and cause considerable morphological damage,leading to change in permeability and the release of cellular contents [38].
In comparing the effects of three oil samples,it can be deduced that the E. coli is more sensitive to the oils derived from flower and aerial parts (P>0.05) rather than the leaf oil (P<0.05). Also,the P. aeroginosa is more sensitive to the oils derived from flower and aerial parts (P>0.05) rather than leaf oil (P<0.05). While,the S. aureus and B. cereus are equally sensitive to the oils derived from leaf,flower and aerial parts (P>0.05).
Conclusions
It can be deduced that the essential oils derived from leaf,flower and aerial parts of the Z. multiflora are fine sources of oxygenated monoterpenes,in particular thymol and carvacrol,with considerable antioxidant and antimicrobial properties. Regarding to the biological properties,these extracts can be suitable candidates for medicinal uses.
References
- Sajed H,Sahebkar A,Iranshahi M. (2013). Zataria multiflora Boiss. (Shirazi thyme)--an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol. 145: 686-698.
- Sharififar F,Moshafi MH,Mansouri SH,Khodashenas M. (2007). In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food Control. 18: 800-805.
- Mahboubi M,Bidgoli FG. (2010). Antistaphylococcal activity of Zataria multiflora essential oil and its synergy with vancomycin. Phytomedicine. 17: 548-550.
- Saei-Dehkordi SS,Tajik H,Moradi M,Khalighi-Sigaroodi F. (2010). Chemical composition of essential oils in Zataria multiflora Boiss from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol. 48: 1562-7.
- Gandomi H,Misaghi A,Akhondzadeh Basti A,et al. (2009). Effect of Zataria multiflora Boiss essential oil on growth and aflatoxin formation by Aspergillus flavus in culture media and cheese. Food Chem Toxicol. 47: 2397-2400.
- Sharififar F,Mirtajadini M,Azampour MJ,Zamani E. (2012). Essential oil and methanolic extract of Zataria multiflora Boiss with anticholinesterase effect. Pak J Biol Sci. 15: 49-53.
- Nakhai LA,Mohammadirad AN,Yasa N,et al. (2007). Benefits of Zataria multiflora Boiss in experimental model of mouse inflammatory bowel disease. Evid Based Complement Alternat Med. 4: 43-50.
- Jafari S,Amanlou M,Borhan-Mohabi K,Farsam H. (2003). Comparative study of Zataria multiflora and Anthemis nobelis extracts with Myrthus communis preparation in the treatment of recurrent aphtous stomatitis. Daru. 11: 1-5.
- Mahmoudabadi AZ,Dabbagh MA,Fouladi Z. (2007). In vitro anti-Candida Activity of Zataria multiflora Boiss. Evid Based Complement Alternat Med. 4: 351-353.
- Ramezani M,Hosseinzadeh H,Samizadeh S. (2004). Antinociceptive effects of Zataria multiflora Boiss fractions in mice. J Ethnopharmacol. 91: 167-170.
- Shokri H,Asadi F,Bahonar AR,Khosravi AR (2006) The Role of Zataria multiflora Essence (Iranian herb) on Innate Immunity of Animal Model. Iran J Immunol. 3: 164-168.
- Shafiee A,Javidnia K. (1997). Composition of essential oil of Zataria multiflora. Planta Med. 63: 371-372.
- Akhondzadeh A,Misaghi A,Khaschabi D. (2007). Growth response and modeling of the effects of Zataria multiflora Boiss essential oil,PH and temperature on Salmonella typhimurium and Staphylococcus aureus. Food Sci Technol. 40: 973-81.
- Aghel N,Moghimipour E,Ameri A. (2007). Characterization of an antidermatophyte cream from Zataria multiflora boiss. Iranian J Pharm Sci. 3: 77-84.
- Fatemi F,Asri Y,Rasooli I,Alipoor ShD,Shaterloo M. (2012). Chemical composition and antioxidant properties of γ-irradiated Iranian Zataria multiflora extracts. Pharm Biol. 50: 232-238.
- Fazlara A,Najafzadeh H,Lak E. (2008). The potential application of plant essential oils as natural preservatives against Escherichia coli O157:H7. Pak J Biol Sci. 11: 2054-2061.
- Goudarzi M,Satari M,Najar Ppiraie SH,Goudarzi M,Bigdeli GR. (2006). Antimicrobial effects of aqueous and alcoholic extracts of theme on enterohmorrhagic Escherichia coli. YAKHT-E. 8: 63-9.
- Misaghi A,Basti AA. (2007). Effects of Zataria multiflora Boiss essential oil and nisin on Bacillus cereus ATCC 11778. Food Control. 18: 1043-9.
- Zargari A. (1990). Medicinal Plants. 1st Edn.,Tehran University Publisher,Iran. 942.
- Gohar AV,Mohammadi A,Sharififar F. (2010). Role of Zataria multiflora boiss essential oil in regulation of MDM2 and ATM genes expression in rat. Asian J Plant Sci. 9: 134-139.
- Walton NJ,Brown DE. (1999). Chemicals from plants: perspectives on plant secondary products. Imperial College Press.
- Burits M,Bucar F. (2000). Antioxidant activity of Nigella sativa essential oil. Phytother Res. 14: 323-328.
- Cuendet M,Hostettmann K,Potterat O. (1997). Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv Chim Acta. 80: 1144-1152.
- Taga MS,Miller EE,Pratt EE. (1984). Chia seeds as a source of natural lipid antioxidant. J Am Oil Chem Soc. 61: 928-931.
- Choi HS,Song HS,Ukeda H,Sawamura M. (2000). Radical-scavenging activities of citrus essential oils and their components: detection using 1,1-diphenyl-2-picrylhydrazyl. J Agric Food Chem. 48: 4156-4161.
- Baron EL,Finegold SM. (1990). Bailey and Scott’s Diagnostic Microbiology,8th Ed.,171–179,The C.V. Mosby Co.,St. Louis,CA.
- NCCLS M2-A7. (2000). Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard,7th Ed.,Clinical and Laboratory Standards Institute,Villanova,PA.
- Mohsenzadeh M. (2007). Evaluation of antibacterial activity of selected Iranian essential oils against Staphylococcus aureus and Escherichia coli in nutrient broth medium. Pak J Biol Sci. 10: 3693-3697.
- Rasooli I. (2007). Food preservation-a biopreservative approach. Food. 1: 111–136.
- Ali MS,Saleem M,Ali Z,Ahmad VU. (2000). Chemistry of Zataria multiflora (Lamiaceae). Phytochemistry. 55: 933-936.
- Basti AA,Misaghi A,Khaschabi D. (2007). Growth response and modelling of the effects of Zataria multiflora Boiss essential oil,pH and temperature on Salmonella typhimurium and Staphylococcus aureus. LWT. Food Sci Technol. 40: 973-981.
- Kavoosi GH,Teixeira da Silva JA. (2012). Inhibitory effects of Zataria multiflora essential oil and its main components on nitric oxide and hydrogen peroxide production in glucose-stimulated human monocyte. Food Chem Toxicol. 50: 3079–3085.
- Mohagheghzadeh A,Shams-Ardakani M,Ghannadi A. (2000). Volatile constituents of callus and flower-bearing tops of Zataria multiflora Boiss. (Lamiaceae). Flavour Fragr J. 15: 373–376.
- Gupta GS,Gupta NL. (1972). Constituents of Zataria multiflora. Phytochemistry. 11: 455–456.
- Shahsavari N,Barzegar M,Sahari MA,Naghdi Badi H. (2008). An investigation on the antioxidant activity of essential oil of Zataria multiflora Boiss in soy bean oil. J Med Plant. 7:56–68.
- Zomorodian K,Saharkhiz MJ,Rahimi MJ,et al. (2011). Chemical composition and antimicrobial activities of the essential oils from three ecotypes of Zataria multiflora. Pharmacogn Mag. 7: 53-59.
- Shafiee A,Javidnia K,Tabatabai M. (1999). Volatile constituents and antimicrobial activity of Zataria multiflora,population Iran. Iran J Chem Chem Eng.18: 1-5.
- Moosavi MH,Basti AA,Misaghi A,Salehi ZT,Abbasifar R. (2008). Effect of Zataria multiflora Boiss essential oil and nisin on Salmonella typhimurium and Staphylococcus aureus in a food model system and on the bacterial cell membranes. Food Res. Int. 41: 1050–1057.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences