Ayahuasca Modifies Amphetamine Self Ingestion and Modifies Anxiety and Locomotor Activity in Adolescent Rats
Godinho AF, Silva MC, Kawashima JD, Horta DF, Anselmo F, De Fraia D
Neurobehavioral Research Laboratory, Center of Toxicological Assistance (CEATOX), Biosciences Institute of Botucatu, Paulista State University (UNESP), Brasil.
- *Corresponding Author:
- Tel: +55 14 3815 3048
E-mail: godinho@ibb.unesp.br
Received date: February 08, 2017; Accepted date: May 09, 2017; Published date: May 16, 2017
Citation: Godinho AF, Silva MC, Kawashima JD, et al. Ayahuasca Modifies Amphetamine Self Ingestion and Modifies Anxiety and Locomotor Activity in Adolescent Rats. Electronic J Biol, 13:2
Abstract
Several reports indicate that the hallucinogen beverage better known as Ayahuasca (AYA) may be utilized for the recuperation of drug abusers. However, there is a lack of scientific evidence for this idea, which led us to use laboratory animals to test it. Amphetamine (AMPH), a substance globally abused principally amongst adolescents, is known to have a high potential to lead to addiction, anxiety, and increases in locomotor activity. The objective of the present work was to experimentally evaluate whether AYA is capable of modifying the self ingestion and behavioral effects caused by AMPH. Adolescent rats were trained to ingest water or AMPH solution (0.6 mg/ml) during a 13 days period, in the absence or presence of AYA treatment (2 ml/kg, gavage). 24 h after the last day of treatment, the rats’ locomotor activity and anxiety behaviors were evaluated using an open field arena (OF) and an elevated plus maze (EPM) apparatus. We observed that the animals have a preference for drinking AMPH, and that AYA prevents this preference. In the EPM tasks, AYA treatment significantly decreased the rise in the percentage of closed arm entries caused by AMPH treatment. In the OF tasks, AYA normalized the hyper-locomotor effect of AMPH, as well as the higher latency to cross the central part of arena and the lower number of center crossings in the arena. Taken together, our findings together indicate that AYA is able to modify AMPH ingestion and its effects. In conclusion, this work presents scientific evidence that ayahuasca can be beneficial for drug abuse users.
Keywords
Ayahuasca; Amphetamine; Drug abuse; Anxiety; Locomotor activity; Behavior.
1. Introduction
Adolescence is associated with an increased sense of adventure, indifference, and risk taking. Adolescents also perceive danger levels to be lower and are prone to anxiety [1]. The age of first encounter with psychoactive compounds is critical, since adolescence have a high probability of being drawn to drugs of abuse and to develop addictions [2].
The reasons for use of drugs of abuse principally in demand by adolescents may be divided into three categories, in accordance with the National Institutes of Health [3]: 1) escape to the awareness of the transience of existence and of the anguish provoked, 2) search for transcendence (contact with supernatural forces and mystical experiences), 3) pursuit of pleasure, which is dominant in modern drug abuse.
Amphetamine (AMPH) is a substance that is considered to be an "illicit drug of abuse". It is being used at increasingly younger ages by young people in pre-adolescence and adolescence stages, suggesting that it is a highly addictive drug [4]. AMPH acts by stimulating the central nervous system through an intensification of norepinephrine signaling. Norepinephrine is a neuro-hormone that activates parts of the sympathetic nervous system.
For a long period of time, AMPH was used to treat depression, epilepsy, and Parkinson's disease. Currently, AMPH is used to treat narcolepsy and attention deficit hyperactivity disorder [5]. In the last few decades, AMPH has also been used in treatments for weight loss, since the drug is temporarily effective in suppressing appetite [6]. In addition, it is common the use of AMPH between students in the middle academic medical [7]. All this is rather worrying, since AMPH is known to be highly addictive and to lead to anxiety and psychomotor activity increases, besides the risk of cardiovascular damages due to the chronic use of amphetamine and analogue drugs [8,9].
Ayahuasca (AYA) is a beverage made from leaves of Psichotria viridis and stems of Banisteriopsis caapi vine [10]. This beverage has psychoactive effects and is largely used by Amerindian cultures for healing, divination, and community bonding, among other uses. More recently, the use of this psychotropic plant tea has experienced an unprecedented expansion worldwide. This expansion in aya use is facilitated by Brazilian churches (principally Santo Daime and União do Vegetal) and has been the object of increasing biomedical research [11].
Previous studies have confirmed that the brew known as AYA contains the beta-carbolines harmine, harmaline and tetrahydroharmine (present in B. caapi) and N,N-dimethyltryptamine (DMT, present in P. viridis) [12]. Interactions between these chemicals constituents and their enzymatic and kinetic activities are most likely responsible for the psychotropic effects observed in individuals using AYA.
Testimonials from AYA users have described the experience as positive and valuable, and some individuals have reported health improvements associated with AYA intake [13]. Recently, Schenberg et al. [14] working with volunteer individuals, offered an interpretation of ayahuasca’s effects based on a cognitive and emotional framework relevant to its ritual use, as well as its potential therapeutic effects. Bogenschutz et al. [15] reviewed the use of classic hallucinogens in the treatment of addictions. Additionally, Domínguez et al. concludes that AYA shows promise as a therapeutic tool by enhancing self-acceptance and allowing safe exposure to emotional events.
The field of study of drug and alcohol dependence and abuse has also shown interest in AYA. This is because individuals with a history of moderate to heavy abuse of alcohol and other substances, such as nicotine, cocaine and amphetamines, including those with functional and social impairments, are frequently observed to abandon the use of these addictive substances after starting the religious use of AYA without having episodes of relapse [16].
Despite various medical and psychological studies on its use, there are few experimental studies on the therapeutic effects of AYA on addiction to drugs of abuse. In previous experiments with rats, we observed that AYA prevented nicotine addiction and the anxiety provoked by abstinence from its use [17].
We therefore hypothesized that AYA can be used as an auxiliary substance for the treatment of drug abusers. To experimentally test this hypothesis, we used adolescent rats to see whether AYA is capable of modulating AMPH self ingestion and its effects on anxiety and locomotor activity.
2. Materials and Method
2.1. Animals
Sixty Wistar male rats of 22 days of age were obtained from the colony housed at Paulista State University and kept under a constant 12 h light/dark cycle. The rats were in an environment with a controlled temperature (21 ± 2°C) until they were used in the experiments. Standard pellet chow (BioBase®, Santa Catarina/SC, Brazil) and tap water were available ad libitum. The institutional Committee of Ethics in Animal Experimentation approved the experimental protocols (309-11). All procedures were performed in accordance with institutional guidelines for animal use and care.
2.2. Experimental groups
Twenty-nine day old (PND 29) animals were divided into four treatment groups (N=15), as follows: Group 1–Control (Ct, animals received only drinking water), Group 2-Amphetamine (AMPH, animals received amphetamine dissolved in their drinking water, 0.6 mg/mL), Group 3-Ayahuasca (AYA, animals received ayahuasca orally, gavage, 2 mL/body weight), Group 4-AMPH+AYA.
2.3. Experimental design
Training of the animals to drink water from two bottles
During a period of nine days (PND 29-37), all animals in the four experimental groups received feed ad libitum and drank water from two glass water bottles of equal shape and size placed next to each other during a restricted period of the day (9:30-11:30 a.m.). This liquid supply scheme was based on experiments of Solomon et al. and has been used in our laboratories in previous experiments to study the effects of AYA on addiction to nicotine [18]. It allows for easy and accurate measurements of the volume of liquid that is ingested.
Administration of amphetamine and ayahuasca to animals
From PND 38 to PND 51, the animals in experimental groups 2 and 4 received two bottles of equal size and shape. One bottle was filled with water and the other contained AMPH solution (0.6 mg/mL, Sigma- Aldrich Brazil). The bottles were placed next to each other during a restricted period of the day (9:30-11:30 a.m.). The position of the bottles was switched daily to avoid position preferences for one part of the cage, what could produce more ingestion of liquid from the bottle in this position. From PND 45 to PND 51, the animals in experimental groups 3 and 4 received 2 ml/kg of body weight AYA by gavage, once a day, between 8:00 and 8:30 a.m. (approximately 1 h before the test).
Throughout the experimental period, the animals received food ad libitum and were weighed at the beginning (PND 38) and the end (PND 51) of the period during which they received AMPH. Between PND 38 and PND 51, the animals were monitored for possible symptoms of AMPH toxicity, and between PND 45 and PND 51, for possible symptoms of AYA toxicity. The behavioral tests were carried out on PND 52.
The general scheme for the training, drug administration and the evaluations that were carried out can be seen in Supplementary data.
The AYA used in the experiments was kindly provided by the Center Spiritual Shamanic Sky of Star Guide in Botucatu, SP., Brazil (CNPJ 08.996.961/0001-00), in the form of the same solution typically ingested by their visitors during spiritual sessions. During these sessions, an adult weighing about 70 kg ingests about 150 ml of solution made from leaves of P. viridis and stems of B. caapi vine.
2.4. Behavioral assessment
Open field test
Open field behavior was assessed using a wooden box measuring 97 × 32.5 cm (diameter × height), as described previously [19]. The box was divided into three concentric circles, which were subdivided by painted black lines into 18 similar spaces. For OF observations, each rat was placed in the center of the arena and was scored on the following parameters for 3 min: ambulation frequency (number of floor units entered with four paws, which reflects locomotor activity) and grooming duration (total time used by the animal for grooming). The following behaviors were considered to be grooming: forepaw vibration, paw licking, washing of nose, face and head, body licking, genital grooming, scratching, and head-shaking. We also assessed passage through the center of the arena, which is an inverse reflection of anxiety [20]. The OF apparatus was carefully cleaned with 5% ethanol before each animal was introduced.
Elevated plus-maze test
Elevated plus-maze behavior was assessed as described previously using an apparatus consisting of two open and two enclosed arms of equal lengths and widths (50 × 10 cm) [21]. The open arms have a 1 cm high Plexiglas edge. The enclosed arms are not entirely enclosed, but rather have walls that extend 40 cm high. The EPM was elevated 50 cm above the floor. Each rat was placed in the center of the elevated plus-maze facing one of the open arms, and the number of entries with four paws and the time spent (in seconds, s) in the open and closed arms were recorded during a 3 min test period. The EPM test is based on the principle that exposure to an elevated and open arm leads to an approach conflict that is considerably stronger than that evoked by exposure to an enclosed maze arm. Thus, the percent of entries and time spent in the closed arms provide a measure of anxiety [22]. The EPM apparatus was carefully cleaned with 5% ethanol before each animal was introduced.
2.5. Statistical analysis
The results were statistically analyzed using GraphPad Instat Software (San Diego, California, USA). Data were compared using the one-way analysis of variance (ANOVA). A Tukey–Kramer post hoc test was used for comparisons between means when ANOVA was significant at the p<0.05 level [23].
3. Results
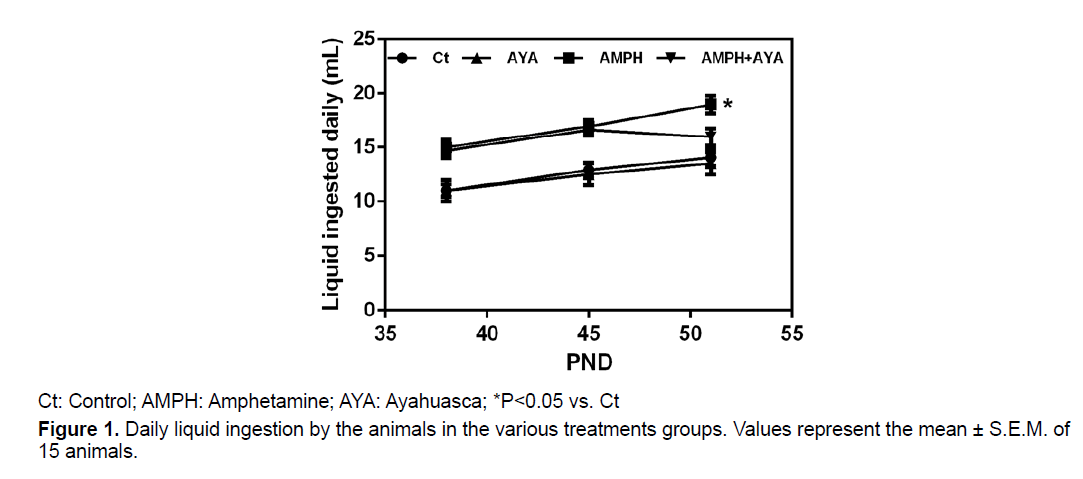
We observed no significant differences (p>0.05) in the water ingestion (PND 38-51) by the animals in the control (Ct) and AYA groups (Figure 1). We observed that the ingestion of AMPH solution (PND 38-51) by animals in the AMPH group was significantly higher (p<0.05) than their ingestion of water, comparing water bottle and AMPH solution bottle of the same animals cage. Animals that received the AMPH solution and were also treated with AYA, significantly decreased (p<0.05) the amount of AMPH solution ingested. This indicates that AYA modulates the animals’ preference for the AMPH solution (Figure 1).
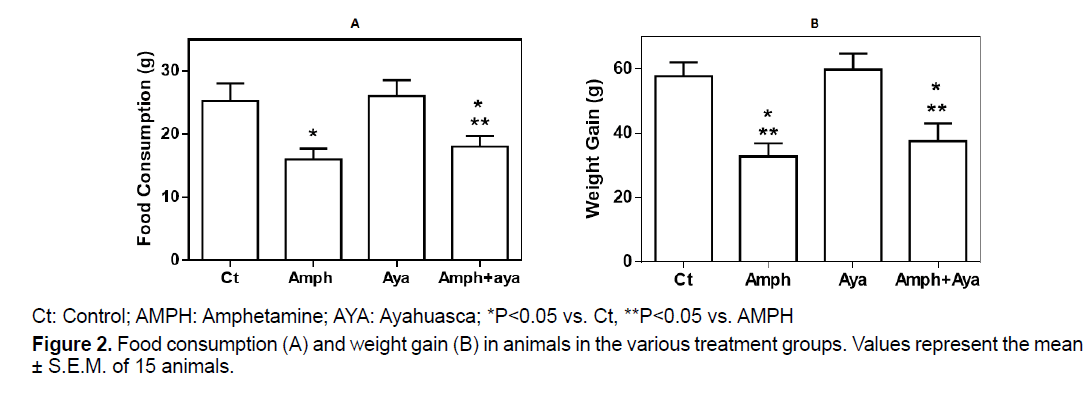
Figure 2A shows that animals receiving amph or AMPH+AYA significantly decreased (p<0.05) their food consumption compared to control animals. Administration of AYA by itself did not significantly change food consumption compared to control animals (p>0.05).
Weight gain was significantly decreased (p<0.05) in animals receiving AMPH or AMPH+AYA compared to control animals (Figure 2B). Administration of only AYA did not change weight gain in the animals (p>0.05).
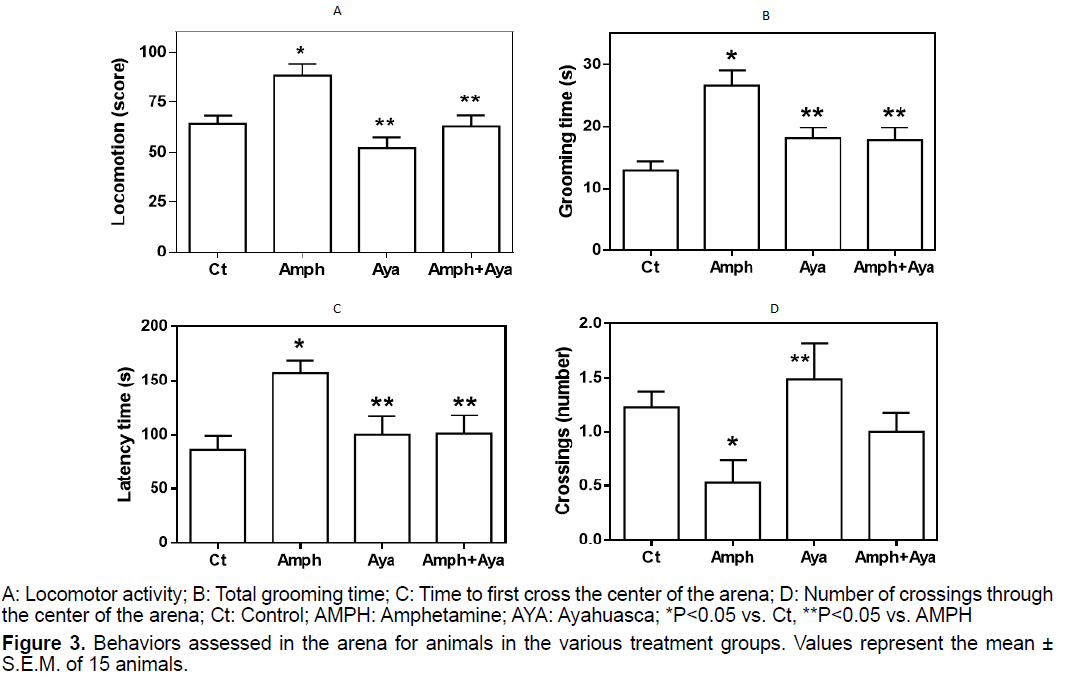
We observed that animals that received AMPH had a significant increase (p<0.05) in locomotor activity compared to control animals (Figure 3A). AYA administration did not significantly change locomotor activity compared to control animals (p>0.05). When animals received AMPH+AYA, their locomotor activity was not different from that of control animals, but was significantly lower (p<0.05) than that of the AMPH group.
We observed that the grooming time was significantly longer (p<0.05) in animals of the AMPH group compared to control animals (Figure 3B). Grooming time was not significantly different of control in animals that received AYA or AMPH+AYA (p>0.05), but it was significantly shorter (p<0.05) when compared to animals of the AMPH group.
Figure 3C shows that animals receiving AMPH have a significant increase (p<0.05) in the latency time to cross the center of the OF arena in relation to control animals. Administration of AYA only did not significantly change (p>0.05) the latency time compared to that of control animals. However, administration of AYA to animals receiving AMPH significantly decreased (p<0.05) the time to first cross the center of the OF arena when compared to animals receiving only AMPH.
We observed that AMPH administration to animals significantly decreases (p<0.05) the number of crossings through the center of the OF arena compared to control animals and animals that received only AYA (Figure 3D). Additionally, administration of AYA to animals that received AMPH normalized the number of crossings through the center of the OF arena.
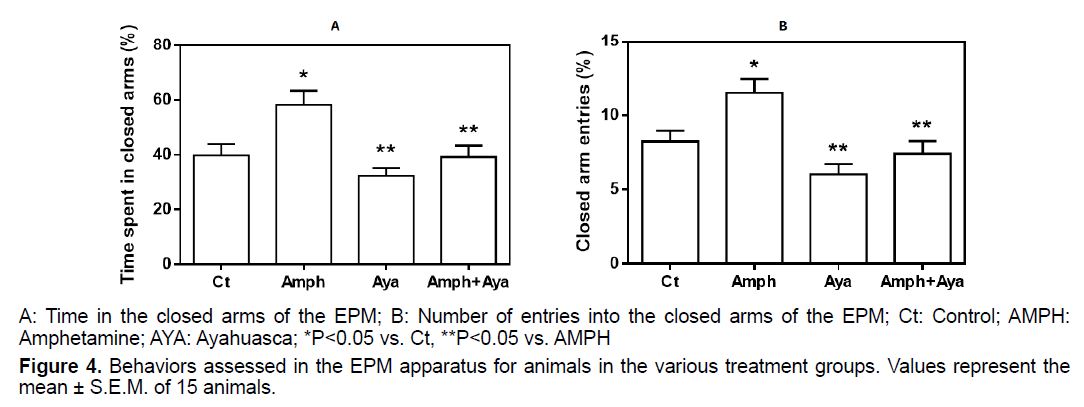
Animals receiving AMPH spent more time (p<0.05) in the closed arms of the EPM compared to control animals (Figure 4A). Administration of AYA only did not significantly change the time spent in the closed arms compared to controls animals (p>0.05). However, AYA administration significantly reduced (p<0.05) the effect of AMPH on the time spent in the closed arms, when compared to animals that received only AMPH.
Animals receiving AMPH had a significant increase in the number of closed arms entries (p<0.05) compared to control animals (Figure 4B). Compared to control, animals that received only AYA did not have significantly alterations in the number of entries into the closed arms. However, administration of AYA significantly reduced (p<0.05) the effect of AMPH on closed arm entries.
4. Discussion
The present study clearly demonstrates that ayahuasca is capable of modifying amphetamine self ingestion and preventing its effects on anxiety and locomotor activity.
Similar to studies on substance use disorders, Oliveira-Lima et al. showed that in mice, the AYA brew (B. caapi and P. viridis combination) not only inhibited early behaviors associated with the initiation and development of ethanol addiction, but was also effective in reversing the behavioral sensitization associated with chronic ethanol administration [24].
Here we show that repeated AMPH treatment (14 days) leads to an increase in locomotor activity, in agreement with a previous study by Lanteri et al. [25]. We also observed that AMPH treatment leads to anxiogenic effects [26].
We demonstrated that in animals receiving both AMPH and AYA the anxiety behavior and locomotor activity were normal. These findings regarding the effects of ayahuasca on the stimulant and anxiogenic effects of amphetamine in the Central Nervous System (CNS) of animals corroborate the results of other researchers. For instance, Schenberg et al. [14] suggest that the effects of AYA at cognitive, affective and emotional levels is relevant and reinforces the suggestion that AYA has therapeutic potential for the treatment of health problems, such as depression, post-traumatic stress disorder, anxiety and drug dependence. These results were surprising and intriguing and lead us to consider the underlying mechanisms in the CNS of animals and humans.
The brain's serotonergic system is known to play an important role in the modulation of anxiety and locomotor activity [27]. Investigations using different strategies for the study of the role of serotonin neurotransmission in specific areas of brain involved in addiction have revealed that the serotonergic system affects anxiety and locomotor activity [28].
DMT has been known to be an endogenous substance in humans and was identified in body fluids a long time ago [29]. DMT is very similar to 5-HT and has a series of potential molecular receptor targets. It interacts with serotonergic neurotransmission due to its structural similarity with the endogenous neurotransmitter serotonin and its affinity for some serotonin receptors. DMT has agonist activity at 5-HT2A and 5-HT1A receptors sites, simulates an excess of serotonin at the receptor level and thus exerts an anxiolytic effect [30,31].
Thus, the effects of ayahuasca are mediated via the potent serotonergic action of DMT in the CNS. More specifically, DMT stimulates 5-HT2A receptors and possibly modulates the action of 5-HT2C receptors [32]. These findings agree with those of Ross [33], who suggested that “converging lines of evidence from pharmacologic, electrophysiologic and behavioral research in animals strongly suggest that activation of cortical 5-Hydroxytryptamine-2A receptors is the most critical step in initiating a cascade of biological events that accounts for serotonergic hallucinogen psychoactive properties”.
Ayahuasca is believed to be harmless for those (including adolescents) drinking it within a religious setting [34]. As there is little research regarding ayahuasca dosage in animal experimentation, we used a dose of 2 mL/kg, which is recommended by Silva et al. [17], as it is comparable to the dose used by humans. This dosage enabled us to observe that AYA reduced the anxiogenic effect of AMPH. In a study in which volunteers ingested aya at a dose of 2 ml/kg, it was observed that the psychoactive effect begins 40 min after the ingestion of AYA and that the duration and the peak intensity of the psychoactive effects coincide with plasma levels of DMT [35,36].
The dose level used in this study is in accordance with the recommendations of the U.S. Environmental Protection Agency for Human Equivalent Dose (HED) calculations used in risk assessment. Based on various studies, the B. caapi and P. viridis versions of AYA appear to have low toxicity and to be reasonably safe in terms of physiological impact when administered to healthy individuals [30]. This enhances the possibility of their use in humans.
On other hand, the actions of the beta-carbolines, harmine and harmaline contributes for the psychoactive effects of AYA. These compounds inhibit the action of monoamine oxidase (MAO), since they are potent reversible inhibitors of this enzyme [37]. Tetrahydroharmine (THH) besides being a weak MAO inhibitor acts as a weak reuptake inhibitor of serotonin [38].
The peripheral inhibition of MAO allows adequate levels of DMT to reach the CNS, causing intense but short sensory and cognitive changes and affection. The principal effects of AYA are a predominant feeling of well-being; a fleeting sensation of apprehension; complex thoughts; new experiences regarding one’s own identity; visible images with eyes closed; visual changes in color, shape, and movement of objects; feeling a more clear and distinct perception of sound; and a change of tact. Such subjective effects start 35 to 40 min after the ingestion of AYA, reaching maximum intensity between 90 and 120 min and ceasing 4 h after administration of the preparation [39,40].
5. Conclusion
This work presents scientific evidence that ayahuasca can be beneficial for drug abuse users.
However, more experimental studies are needed to clarify the neural and physiological mechanisms that ayahuasca modulates so that it can be suggested as an auxiliary instrument in the treatment of chemical dependency in the future.
7. Acknowledgement
Authors are grateful to the Center Spiritual Shamanic Sky of Star Guide, in Botucatu, SP., Brazil (CNPJ 08.996.961/0001-00) by the ayahuasca donation.
References
- Slotkin TA, Stadler A, Skavicus S, et al. (2016). Adolescents and adults differ in the immediate and long-term impact of nicotine administration and withdrawal on cardiac norepinephrine. Brain Res Bull. 122. 71-75.
- Spear LP. (2000). The adolescent brain and age-related behavioral manifestations. Neurosc Biobehav Rev. 24: 417ÃÆâÃâââ¬Ãâââ¬Å463.
- National Institute on Drug Abuse. (2014). Principles of adolescent substance use disorder treatment: A research-based guide NIH publication number 14-7953.
- Teixeira AG, Costa VM, Feio RA, et al. (2015). The neurotoxicity of amphetamines during the adolescent period. Intern J Develop Neurosc. 41: 44-62.
- Yoshida T. (1997). Use and Misuse of Amphetamines: An International Overview. In H. Klee (Ed.), Amphetamine Misuse: International Perspectives on Current Trends, Harwood Academic Publishers. Amsterdam. 1-16.
- Wilens TE, Adler LA, Adams J, et al. (2008). Misuse and diversion of stimulants prescribed for ADHD: A systematic review of the literature. J Am Acad Child Adolesc Psych. 47: 21-31.
- Da Silveira DX, Rosa LO, Di Pietro M, et al. (2008). Evolutional pattern of drug use by medical students. Addic Behav. 33: 490-495.
- Hanson GR, Rau KS, Fleckenstein AE. (2004). The methamphetamine experience: A NIDA partnership. Neuropharmacology. 47: 92-100.
- Markham CM, Yang M, Blanchard RJ, et al. (2006). Effects of D-Amphetamine on defensive behaviors related to fear and anxiety. Pharmacol Biochem Behav. 83: 490-499.
- DomÃÆÃâÃâÃÂnguez EC, Soler J, Elices M, et al. (2016). Ayahuasca: Pharmacology, neuroscience and therapeutic potential. Brain Res Bull.
- Frood A. (2015). Ayahuasca psychedelic tested for depression - Pilot study with shamanic brew hints at therapeutic potential. Nature. 1-5.
- McIlhenny EH, Pipkin KE, Standish LJ, et al. (2009). Direct analysis of psychoactive tryptamine and harmala alkaloids in the Amazonian botanical medicine ayahuasca by liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromat A. 1216: 8960-8968.
- Loizaga-Velder A. (2013). A psychotherapeutic view on the therapeutic effects of ritual ayahuasca use in the treatment of addiction. MAPS Bull. 23: 36-40.
- Schenberg EE, Alexandre JFM, Filev R, et al. (2015). Acute biphasic effects of ayahuasca. PLoS ONE. 10: e0137202.
- Bogenschutz MP, Johnson MW. (2016). Classic hallucinogens in the treatment of addictions. Prog Neuro-Psychopharmacol Biol Psych. 64: 250-258.
- McKenna DJ. (2004). Clinical investigations of the therapeutic potential of ayahuasca: Rationale and regulatory challenges. Pharmacol Ther. 102: 111-129.
- Silva MC, Figueiredo CG, Godinho AF. (2008). Experimental study of Ayahuasca effect on nicotine addiction in adolescent Wistar rats. J Venom Anim Tox including Trop Dis. 14: 856.
- Salomon L, Lanteri C, Glowinski J, et al. (2006). Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Nat Acad Sci Unit States Am. 103: 7476-7481.
- Broadhurst PL. (1960). Experiments in psychogenetics, In H.J. Eysenk (Ed.), Experiments in personality, Rutledge and Keagan Paul, London. 31-61.
- Prut L, Belzung C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 463: 3-33.
- Pellow S, File SE. (1986). Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus maze: A novel test of anxiety in the rat. Pharmacol Biochem Behav. 24: 525-529.
- Carobrez AP, Bertoglio LJ. (2005). Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years. Neurosci Biobehav Rev. 29: 1193-1205.
- Snedecor GW, Cochran WG. (1991). Statistical Methods, 8th edn, Wiley, Iowa.
- Oliveira AJL, Santos RD, Hollais AW, et al. (2015). Effects of ayahuasca on the development of ethanol-induced behavioral sensitization and on a post-sensitization treatment in mice. Physiol Behav. 142: 28-36.
- Lanteri C, Salomon L, Torrens Y, et al. (2015). Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology. 33: 1724-1734.
- Vanderschuren LJMJ, Schoffelmeer ANM, Mulder AH, et al. (1999). Dopaminergic mechanisms mediating the long-term expression of locomotor sensitization following pre-exposure to morphine or amphetamine. Psychopharmacology. 143: 244-253.
- Blazevic S, Colic L, Culig L, et al. (2012). Anxiety-like behavior and cognitive flexibility in adult rats perinatally exposed to increased serotonin concentrations. Behav Brain Res. 20: 175-181.
- Pum ME, Huston JP, MÃÆÃâÃâüller CP. (2009). The role of cortical serotonin in anxiety and locomotor activity in Wistar rats. Behav Neurosci. 123: 449-54.
- Ciprian-Olivier J, Cetkovich BMG. (1997) Altered consciousness states and endogenous psychoses: a common molecular pathway? Schizoph Res.28: 257-265.
- Bouso JC, GonzÃÆÃâÃâález D, Fondevila S, et al. (2012). Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of ayahuasca: A longitudinal study. PLoS ONE. 7: 1-2.
- Jacob MS, Presti DE. (2005). Endogenous psychoactive tryptamines reconsidered: an anxiolytic role for dimethyltryptamine. Med Hypoth. 64: 930-937.
- Fantegrossi WE, Harrington AW, Kiessel CL, et al. (2006). Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 83: 122-129.
- Ross S. (2012). Serotonergic hallucinogens and emerging targets for addiction pharmacotherapies. Psych Clin North Am. 35: 357-374.
- Da Silveira DX, Grob CS, De Rios MD, et al. (2005). Ayahuasca in adolescence: A preliminary psychiatric assessment. J Psych. Drugs. 37: 129-133.
- Callaway J, McKenna DJ, Grob CS, et al. (1999). Pharmacokinetics of Hoasca alkaloids in healthy humans. J Ethnopharmacol. 65: 243-256.
- https://www.epa.gov/raf/publications/interspecies-extrapolation.htm
- Riba J, RodrÃÆÃâÃâÃÂguez FA, Urbano G, et al. (2001). Subjective effects and tolerability of the South American psychoactive beverage ayahuasca in healthy volunteers. Psychopharmacology. 154: 85-95.
- McKenna DJ, Callaway JC, Grob CS. (1998). The scientific investigation of ayahuasca: A review of past and current research. Heffter Rev Psych Res. 1: 65-76.
- Grob CS, McKenna DJ, Callaway JC, et al. (1996). Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. J Nerv Mental Dis. 184: 86-89.
- Vollenweider FX, Geyer MA. (2001). A systems model of altered consciousness: Integrating natural and drug-induced psychoses. Brain Res Bull. 56: 495-507.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences