Antibiotic Resistant Bacteria in Mud of Shrimp Farming Ponds and Bacterial Degradation of Antibiotic
Jatindra Nath Bhakta, Yukihiro Munekage
Department of Environmental Engineering, Faculty of Agriculture, Kochi University, B200 Monobe, Nankoku, Kochi 783-8502, Japan
Abstract
An investigation was conducted to determine the occurrence, resistant efficiency of bacteria collected from four shrimp farming zones, Vunh Tau (VT), Nha Trang (NT), Da Nang (DN) and Hue (HU) of Viet Nam against five commonly applied antibiotics [sulphamethoxazole (SMX), trimethoprim (TMP), norfloxacin (NFC), amoxillin (AXC) and streptomycin (SMC)] and bacterial degradation of antibiotic. Mud samples were employed to examine the resistant population and antibiotic resistant assay of bacteria using spread plate technique and disc diffusion method, respectively. Significantly high resistant bacterial populations were found in SMX (76–296 X 103 cfu/g), TMP (39–309 X 103 cfu/g), AXC (135–154 X 103 cfu/g) and SMC (93–239 X 103 cfu/g), whereas NFC showed a lowest count (0–17 X 103 cfu/g) in all sampling stations. Antibiotic resistant of bacteria was also strong as no clear zones were found in SMX and TMP up to 6 μg/ml and in AXC upto 4 μg/ml concentrations among five antibiotics. Bacterial degradation of the antibiotic (TMP, 0.098 μg/ml/day) clearly pronounced that rate of antibiotic degradation governed by the bacterial resistant ability against the antibiotic. It may also be indicated that unutilized excessive antibiotic residues in mud of shrimp ponds lead to immeasurable loss in the microbial profile by acquiring high degree of resistant efficiency against SMX, TMP and AXC antibiotics in aquaculture environment. Therefore, avoidance of antibiotics application in aquaculture system would be the priority step to minimize and control the severe effects of antibiotic residues and resistant microbial population for the conservation of environment.
Keywords
Shrimp pond; mud; antibiotics; resistant bacteria; antibiotic degradation.
1. Introduction
Indiscriminate application of antibiotic agents against different target organisms leads to unimaginable negative impact in aquatic environments. Presently, antibacterial as well as multiple antibacterial resistance (MAR) are the tremendous accelerating and accumulating problem of the aquaculture environment affecting the ability to treat infectious diseases and presents a major threat to public health. Worldwide increasing demand of shrimp triggers the intensification of farming practices to maximize the production as well as profit resulting in disease outbreak which is among the biggest concerns in shrimp [1]. Intensive shrimp culture requires a large amount of shrimp food and antibiotics to increase production and protect shrimp from diseases [2]. Consequently, a large portion of unused feeds and antibiotics contaminate the water as wastes, causing water pollution [3] as well as a major part is also deposited on the bottom mud of shrimp ponds which is responsible for the development of antibiotic resistant microbial community in the culture environment. Presence of antibacterial residues has been reported by several studies [4-6] in fish farms and it has been known a number of antibiotics persist in the sediment and aquatic environment for several months following administration [7-11]. McPhearson et al. observed that individual and multiple antibiotic resistances were associated with antimicrobial use [12]. Researchers also reported that bacteria were resistant to oxolinic acid, trimethoprim and sulfamethoxazole [13-15]. Herwig et al. suggested that antimicrobials may increase the level of resistance in bacteria in the surrounding environments [6]. Abraham et al. was able to isolate V. harveyi strains from diseased shrimp that are resistant to ampicillin, chlorotetracycline, ceprofloxacin, erythtromycin, furazolidone, gentamycin, nalidixic acid, neomycin, nitrofurantoin, nitrofurazole, novobiocin, ofloxacin, oxytetracycline, penicillin G, polymyxin B, rifampicin, streptomycin, sulphasomodine, sulphamethezole, and sulphafurazole [16].
To produce tiger shrimp (Penaeus monodon), huge number of shrimp culture ponds have grown significantly over the last ten years in Viet Nam to meet the increasing market demand. Though few researches concerning presence of antibiotic resistant bacteria in aquafarming system in Viet Nam were performed so far, but the information regarding bacterial resistant efficiency and bacterial degradation of antibiotics are very scanty. Therefore, the present study has attempted to determine the occurrence, efficiency of resistant bacterial population against some commonly used antibiotics in four shrimp farming coastal zones of Viet Nam and bacterial degradation of antibiotics.
2. Materials and Methods
2.1 Study sites
Present investigation considered four coastal shrimp farming zones; Vunh Tau, Nha Trang, Da Nang and Hue located in Viet Nam. Vunh Tau (VT) (10° 21' 0" N and107° 4' 0" E) and Nha Trang (NT) (12° 13' 40" N and 109° 11' 38" E) are situated on the coast of the southeastern region whereas Da Nang (DN) (16° 2' 38" N and 108° 11' 58" E) and Hue (HU) (16° 28' 0" N and 107° 36' 0" E) are also located on the coast of the South China Sea (Figure 1).
2.2 Sampling
Mud samples (0–5mm) were collected aseptically from randomly selected five ponds of each coastal zone, mixed properly, pooled into one and preserved in sterile plastic bottle (250 ml).
2.3 Count of resistant population
The suspension of mud was prepared by mixing 1 g of wet mud in 99 ml sterile seawater. Aliquots of ten fold dilution 10-3 to 10-4 were prepared in sterile seawater water. Five types of antibiotics, sulphamethoxazole (SMX), trimethoprim (TMP), norfloxacin (NFC), amoxillin (AXC) and streptomycin (SMC) were used in nutrient marine agar media [17] @ 5μg/ml to enumerate viable counts of antibiotic resistant bacterial colony using conventional spread plate technique under aerobic conditions at an incubation temperature of 35°C for 48h. Each dilution of the sample was plated in triplicate and arithmetical mean of colony forming unit (cfu) of the three counts was used for statistical analysis.
2.4 Isolation and preparation of bacterial culture
Aseptically, representative colonies of four coastal zones grew in each antibiotic containing agar media which were transferred into a 100 ml sterilized conical flask of nutrient broth by a loop, cotton plugged and placed in incubator overnight at 37 °C with shaking condition. After 12 to 18 h of incubation, the cultures were diluted with sterile seawater to bring the final inoculum size approximately 105 to 106 cfu/ml [18,19].
2.5 Antibiotic resistant assay
One millilitre of cultured bacteria was inoculated on agar plate for preparation of loan of bacteria used for the test of antibiotic resistant assay following the standard methods of paper disc diffusion assay. Solutions of different concentrations (1, 2, 4, 6, 8, 10, 20 and 50 μg/ml) of each antibiotic were prepared and 70μl of solution was pipetted onto each disc (8 mm, Advantec, Toyo Roshi Kaisha Ltd.). The discs were then transferred on to the bacterium seeded/inoculated agar plate. Each inoculum was employed in triplicate and incubated at 37 °C for 24 h. Antibiotic resistant efficiency was assessed based on measuring the diameter (mm) of the clear zone around the disc.
2.6 Bacterial degradation of antibiotics
Degradation study was conducted on one lower molecular weight antibiotic, trimethoprim (TMP) and other higher molecular weight antibiotic, streptomycin (SMC). One millilitre of pre-incubated bacterial culture of the collected mud samples (mixed of four zones) were mixed with 9 ml nutrient broth, incubated at 37°C for 18 h to get fresh bacterial culture and adjusted to a density of 108 cells/ml. Each antibiotic was mixed in 10 ml of fresh bacteria culture and control (only broth) groups @ 2μg/ml in triplicates and incubated at 37°C. Samples (1 ml) were collected on 3rd and 7th day, filtered by 0.25 μm filter (Advantec, Tokyo, Japan) and used for measuring the concentration by HPLC.
2.7 Statistical analysis
All data were subjected to one way analysis of variance (ANOVA) in the general linear model using the SPSS 10.05 statistical package. The statistical package (EASE, M-STAT) was used in the computer to perform the analyses of least significance difference (LSD). The level of acceptable statistical significance was specified at P < 0.05.
3. Results
3.1 Resistant bacterial population
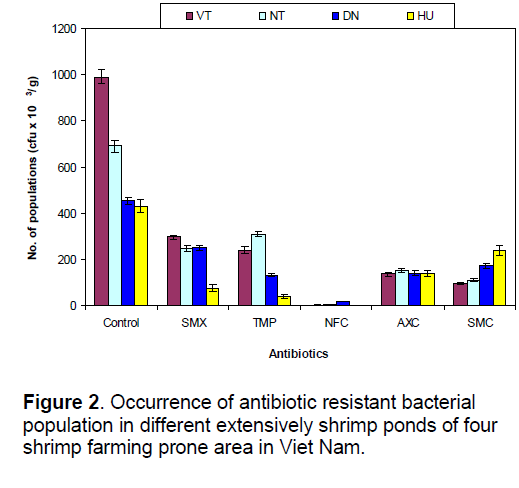
The population counts of heterotrophic bacteria varied from 43.1 to 99 X 104 cfu/g in control group having no antibiotics, whereas a remarkable variation (0 to 389 X 103 cfu/g) was observed in all antibiotic treated groups and exhibited the following order of variation in the mean count of four sampling stations: SMX>TMP>SMC>AXC>NFC (ANOVA, P < 0.05) (Figure 2). SMX resistant bacterial population was maximum (296 X 103 cfu/g) and minimum (76 X 103 cfu/g) in VT and HU, respectively. Resistant bacterial count against TMP was highest (309 X 103 cfu/g) in NT and showed the following order of variation: NT>VT>DN>HU (ANOVA, P < 0.05). Number of NFC resistant bacterial population was lowest (0–17 X 103 cfu/g) among the five antibiotics employed in the all sampling stations. NT (154 X 103 cfu/g) and HU (239 X 103 cfu/g) exhibited the elevated resistant bacterial counts against the AXC and SMC (Fig. 2). Above results clearly revealed that bacterial population in the mud of investigated coastal shrimp farming ponds acquired resistant properties against five tested antibiotics. The mean resistant bacterial populations were 217.5, 179.5, 5, 142 and 153.5 X 103 cfu/g in SMX, TMP, NFC, AXC and SMC, respectively, whereas total mean heterotrophic bacterial count was 64.1 X 104 cfu/g.
3.2 Antibiotic resistant assay
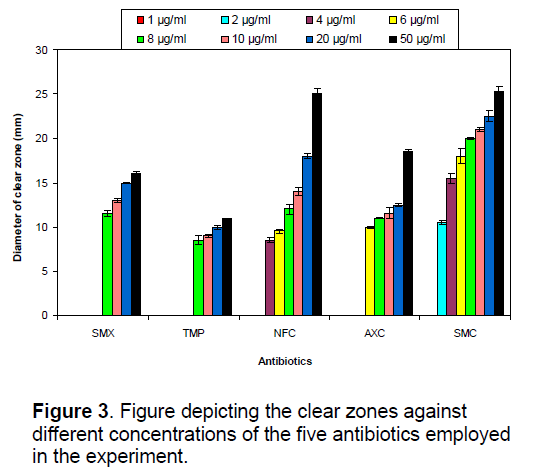
In vitro, disc diffusion assay of isolated resistant bacteria of four coastal areas against the five antibiotics showed a significant difference in the clear zones developed around the discs (ANOVA, P<0.05) (Fig. 3). Though no clear zones around the discs were observed in four lower concentrations (1–6 μg/ml) but the value of clear zone increased from 11.5 to 16 mm and 8.5 to 11 mm with increasing concentration in SMX and TMP, respectively. Clear zones were started at the antibiotic concentration doses 2 and 4 μg/ml and ranged from 8.5 to 25 mm and 10 to 18.5 mm in NFC and AXC, respectively. Excepting lowest concentration, SMC pronounced clear zone varying from 10.5 to 25.3 mm in remaining all concentrations of antibiotics. A clear increasing trend was observed in the clear zone around the disc with increasing concentration in all antibiotics (Figure 3).
3.3 Bacterial degradation of antibiotics
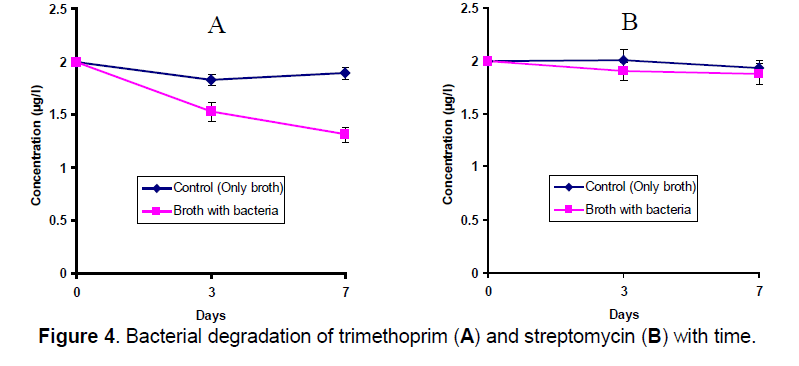
Though a significant bacterial degradation was observed in TMP (ANOVA, P<0.05) but no remarkable difference was found between the bacterial treated and control groups in SMC (Figure 4). Degradation rate was 0.098 μg/ml/day in TMP.
4. Discussion
Above results demonstrated that 34, 28, 0.07, 22 and 23.94% of total counted heterotrophic bacteria developed as antibiotic resistant against the SMX, TMP, NFC, AXC and SMC antibiotics, respectively. This proposition indicates that residues of the huge and frequent applied antibiotics in the shrimp farm are responsible for emerging the antibiotic resistant properties of bacteria. The residues of antibacterial agents may affect the sedimentary microbial community and introduce antibiotic resistance in the bacteria [20,21]. Furthermore, it can be inferred that the application of SMX and TMP antibiotics were maximum followed by AXC and SMC, whereas NFC may not be applied or may be applied with very little amount in the investigated areas as very few percentage of bacteria acquired resistant ability against NFC. In this context, it was found that VT and NT were predominated with SMX and TMP resistant bacteria as well as SMX and SMC resistant bacteria more pronounced in DN, whereas HU showed the abundance of SMC and AXC resistant bacteria. These results implied that the zonewise specific antibiotic resistant specificity is only due to the reason of routinely used specific antibiotics in the management scheduled of farm in different investigated regions.
Antibiotic resistant of the SMX and TMP resistant bacteria were strong upto antibiotic concentration 6 μg/ml as well as AXC resistant bacteria upto 4 μg/ml as no clear zones were observed, whereas clear zone started at 2 and 1 μg/ml in NFC and SMC antibiotics, respectively (Fig. 3). It, therefore, may also be signified that bacterial populations of investigated shrimp ponds acquired high degree of resistant efficiency against SMX, TMP and AXC antibiotics than that of the remaining two (NFC and SMC) antibiotics which indicating that farmers of the investigated ponds were supposed to be applied SMX, TMP and AXC with high concentration during long periods rather than the other tested antibiotics. Munekage et al. [22] and Le and Munekage [23] reported the presence of norfloxacin, oxalinic acid, trimethoprim and sulphamethoxazole in sediments of Thai Binh, Nam Dinh, Can Gio–Ho Chi Minh City and Ca Mau mangrove-based shrimp farming Province of Viet Nam.
Results of antibiotic degradation revealed that since bacterial communities of investigated zones acquired higher resistant efficiency against trimethoprim compared to streptomycin, therefore trimethoprim degradation potentiality of the bacteria was greater than the streptomycin. It may also be proposed that antibiotics of the low molecular weight degrade more easily rather than the high molecular weight antibiotic.
5. Conclusions
In conclusion, it can be inferred that strong resistant properties have developed in bacterial populations against Sulphamethoxazole (SMX), Trimethoprim (TMP), Amoxillin (AXC) and Streptomycin (SMC) among the five tested antibiotics in the four shrimp farming area of Viet Nam. Inspite of that it may also be concluded resistant efficiency of SMX, TMP and AXC antibiotic resistant bacteria were greater over the rest two antibiotics. Bacterial degradation of the antibiotic also clearly pronounced antibiotic degradation governed by the acquired bacterial resistant ability against the antibiotic as well as molecular weight of antibiotic. Moreover, this study would be a platform of further study concerning the development of potentially antibiotics degrading probiotic bacteria from resistant bacterial population to improve the antibiotic contaminated environment. On account of the above results it is obvious, unutilized excessive antibiotic residues in the mud of shrimp ponds have invited an immeasurable loss in the microbial profile of aquaculture environment by acquiring high degree of resistant efficiency against SMX, TMP and AXC antibiotics resulting in the negative feed back for controlling the shrimp disease. Therefore, avoidance of antibiotics application to control the disease outbreak in aquaculture system adopting other alternative control measure would be the priority step to prevent the generation of antibiotic residues and resistant microbial population in order to conserve the total environment.
Acknowledgements
Authors are grateful to Govt. of Japan for sponsoring the JSPS research grant (No. 20380181) to carry out the present study. Dr. Bhakta is also grateful to Kochi University for providing the position of Researcher Faculty.
References
- Uyen N.V. (2002) Viral diseases in shrimp a challenge to the biotechnologists in Vietnam, Institute for tropical biology, Ho Chi Minh, Vietnam.
- EJF (2003) Risky business: Vietnamese shrimp aquaculture–impacts and improvements, Environmental Justice Foundation, London, UK.
- Le T.X., Munekage Y., Phan D.A.T., Quan D.Q.T., et al. (2003, May) The environmental quality of shrimp ponds in mangrove areas. In: Thirteenth International Offshore and Polar Engineering Conference Proceedings, Honolulu, HI, USA, pp. 255-262.
- Weston D.P. (1996) Environmental considerations in the use of antibacterial drugs in aquaculture. In: Aquaculture and Water Resources Management (Baird D., et al. ed.) Blackwell Science, pp.140-165.
- Capone D.G., Weston D.P., Miller V., Shoemaker, C., et al. (1996) Antibacterial residues in marine sediments and invertebrates following chemotherapy in aquaculture. Aquaculture, 145: 55-75.
- Herwig R.P., Gray J.P., Weston D.P., et al. (1997) Antibacterial resistant bacteria in surficial sediments near salmon net-cage farms in Puget Sound, Washington. Aquaculture, 149: 263–283.
- Bjorklund H.R., Bbergh C.M.I., Bylund G., et al. (1991) Residues of oxolinic acid and oxytetracycline in fish and sediments from fish farms. Aquaculture, 97: 85-96.
- Lunestad B.T., Samuelsen O.B., Fjelde S., Ervik A., et al. (1995) Photostability of eight antibacterial agents in seawater. Aquaculture, 134(2): 17-25.
- Pouliquen H., Le B. (1996) Sorption of Oxolinic acid and oxytetracycline to marine sediments. Chemosphere, 33(5): 801-815.
- Hirsch T.T., Haberer K., Kratz K.-L., et al. (1999) Occurrence of antibiotics in the aquatic environment. Sci. Total Environ., 225(1–2): 109-118.
- iranda C.D., Zemelman R. (2002) Bacterial resistance to oxytetracycline in Chilean salmon farming. Aquaculture, 212(1–4): 31-47.
- McPhearson Jr. R.M., DePaola A., Zywno S.R., Motes Jr. M.L., Guarino A.M., et al. (1991) Antibiotic resistance in Gram-negative bacteria from cultured catfish and aquaculture ponds. Aquaculture, 99(3/4): 203-211.
- Hansen P.K., Lunestad B.T., Samuelsen O.B., et al. (1992) Effects of oxytetracycline, oxolinic acid, and flumequine on bacteria in an artificial marine fish farm sediment. Can. J. Microbiol., 38: 1307-1312.
- Nygaard K., Lunestad B.T., Hektoen H., Berge J.A., Hormazabal V., et al. (1992) Resistance to oxytetracycline, oxolinic acid and furazolidone in bacteria from marine sediments. Aquaculture, 104: 31-36.
- Holmoström K., Gräslund S., Wahlström A., Poungshompoo S., Bengtsson B.E., Kautsky N., et al. (2003) Antibiotic use in shrimp farming and implications for environmental impacts and human health. Int. J. Food Sci. Technol., 38: 255-266.
- Abraham T.J., Manley R., Palaniappan R., Dhevendaran K., et al. (1997) Pathogenicity and antibiotic sensitivity of luminous Vibrio harveyi isolated from diseased penaeid shrimp. J. Aquat. Trop., 12(1): 1-8.
- APHA (1989) Standard method for examination of water and waste water (17th ed.). America Public Heath Association, Washington, DC.
- Lee S.-H., Chang K.-S., Su M.-S., Huang Y.-S., Jang H.-D., et al. (2007) Effects of some Chinese medicinal plant extracts on five different fungi. Food Control, 18: 1547-1554.
- Trakranrungsie N., Chatchawanchonteera A., Khunkitti W., et al. (2007) Ethnoveterinary study for antidermatophytic activity of Piper betle, Alpinia galanga and Allium ascalonicum extracts in vitro. Res. Vet. Sci., 84(1): 80-84.
- Hektoen H., Berge J.A., Hormazabal V., Yndeslad M., et al. (1995) Persistence of antibacterial agents in marine sediments. Aquaculture, 133: 175-184.
- Tendencia E.A., De la Pena L.D. (2001) Antibiotic resistance of bacteria from shrimp ponds. Aquaculture, 195: 193-204.
- Munekage Y., Le T.X., Natsukawa Y., Iwasaki N., et al. (2002) Distribution of persistent chemicals in estuary and its accumulation in plankton. Cost. Eng., 49: 1096-1100 (in Japanese).
- Le T.X., Munekage Y. (2004) Residues of selected antibiotics in water and mud from shrimp ponds in mangroves areas. Mar. Pollut. Bull., 49(11-12): 922–92.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences