Antibacterial Activity of Raw and Processed Honey
Abhishek Chauhan, Vimlendu Pandey, K. M. Chacko and R. K. Khandal
Department of Microbiology, Shriram Institute for Industrial Research 19, University road, Delhi-110007, India
- Corresponding Author:
- R. K. Khandal
Department of Microbiology
Shriram Institute for Industrial Research 19
University road, Delhi-110007, India
Tel: +91(0)11-27667267
Fax: +91(0)11-27667676
E-mail: micro@shriraminstitute.org
Abstract
The antibacterial activity of raw and processed honey was carried out on the extracts of honey using solvents such as methanol, ethanol and ethyl acetate and compared it with the popular antibiotics. The inhibitory action of extracts of honey were evaluated against six bacterial strains, Gram-positive bacteria viz., Staphylococcus aureus, Bacillus subtilis, Bacillus cereus and Gram-negative bacteria, Escherichia coli, Pseudomonas aeruginosa and Salmonella typhi by agar well diffusion method. The extracts of raw and processed honey showed the zone of inhibition ranged from 6.94 mm to 37.94 mm. Methanol extract was more potent and showed a stronger antibacterial activity, followed by ethanol and ethyl acetate in the same order. Further, the residues remaining after extraction with solvents showed no bactericidal effect indicating that only extracts exhibit the antibacterial activity. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) were evaluated by Macro broth dilution method. The most susceptible bacteria were Salmonella typhi, E. coli. and Pseudomonas aeruginosa. MIC and MBC values of extracts were found in the range of 0.625-5.000 mg/ml. The solvent extract of honey showed better antibacterial behaviors against Gram-negative bacteria than the standard antibiotics such as tetracycline and Ciprofloxacin. Infact the solvent extracts of honey were found to be bactericidal against P. aeruginosa for which even tetracycline was found ineffective.
Keywords
Agar well diffusion assay; Honey; Minimum Inhibitory Concentration; Minimum Bactericidal Concentration
Introduction
According to World Health Organization estimates, some 80 percent of people living in developing countries rely on harvested wild plants for their primary health care. In the developing world the use of antibiotics constitute a sizable fraction of medicines consumed. However, because of the microbes turning resistant especially to the synthetic antibiotics in use, the effectiveness of the antibiotics has been diminishing [1]. This type of resistance to antimicrobial agent has already become a serious issue in many areas of the world especially in developing countries [2,3]. Over the years, antibacterial substances from natural resources have been identified and exploited for this purpose. Various plants and their extracts have already been in use as per the Indian system of medicines (Ayurveda) for the treatments requiring antimicrobial activity. One of the popular antimicrobial natural substances described in Ayurveda as a potent medicine for several uses is honey.
Honey is the natural sweet substance from nectar or bossoms or from the secretion of living parts of plants or excretions of plants, which honey bees collect, transform, and combine with specific substances of their own to ripen and mature [4]. It is also defined as the nectar and saccharine exudation of plants, gathered, modified and stored as honey in the honeycomb by honeybees. Honey is widely used in traditional medicine throughout the world. However, it has a limited use in modern medicine due to lack of scientific support [5]. Ayurveda, an ancient Indian System of health care treats as food for health while recommending it as a medicine ancient medicine for some conditions using it externally as well as orally. It is known to cure anemia and improves calcium fixation in infants. Honey also reduces and cures eye cataracts and conjunctivitis and applied honey directly to the eye cures various diseases of the cornea [6]. There are many reports of honey being very effective as dressing of wounds, burns, skin ulcers and inflammations. The antibacterial properties of honey speed up the growth of new tissue to heal the wound [7]. The bactericidal effect of honey is reported to be dependent on concentration of honey used and the nature of the bacteria [8,9]. The bactericidal action is reportedly ascribed neither the normal acidity of honey, nor to its high sugar content, enzymes, nitrogenous or other compounds, accumulation of hydrogen peroxide, which is produced by natural glucose oxidize system in honey [7,10,11]. Farouk et al. [12] found that there were inhibitory effects on Gram-positive and Gram-negative strains both standard test organisms and clinical isolates from inflamed wounds.
Honey consists of various constituents such as water, carbohydrates, proteins, vitamins, amino acid, energy and minerals. Besides the major ones, there must also be several minor constituents in honey, which may be playing a key role in determining the antimicrobial behaviour of honey. In the past, antimicrobial activity of honey had been reported only by using aqueous solution of honey. It is said that honey possesses antibacterial property but it is not clear whether it is the bulk honey or some fraction of it. Considering the fact that their might be some specific constituents which may be contributing to the antimicrobial behaviour, it was decided to carryout the studies using different solvents. The present study therefore deals with the constituents in the different solvents followed by evaluation of extract for their antimicrobial behaviour against certain species of bacteria.
Materials and Methods
Collection of Honey Sample
Honey samples two each raw and processed were collected from rural areas of western U.P., India and from a local honey processor respectively.
Microbial Strains
A total of six bacterial strains including (a) Gram-positive (Staphylococcus aureus MTCC 737, Bacillus subtilis MTCC 736, Bacillus cereus MTCC 430) and (b) Gram-negative bacteria (Pseudomonas aeruginosa MTCC 741, Escherischia coli MTCC 1687 and Salmonella typhi MTCC 531) were obtained from the Microbial Type Culture Collection (MTCC) IMTECH, India.
Tetracycline and Ciprofloxacin
Tetracycline hydrochloride capsule, Mfg. By. -Cipla ltd. and Ciprofloxacin, Mfg. By-Nicolas Piramal India Ltd. were obtained from local pharmacy store. Both the antibiotics were used in 5 μg/ml concentration against every bacterial strains.
Extraction
Extraction of both raw and processed honey was performed by using organic solvents, for this, 10g of honey was taken in a centrifuge tube with 25ml of solvent and then mixed well by vortexing and shaking with hands for about 30 minutes. This was centrifuged at 3000 rpm for 10 minutes at 25ºC. Supernatant was collected from each centrifuged tube in a round bottom flask by filtration. The resulting supernatant was dried under nitrogen gas with a temperature of 50ºC. Methanol free content of the flask were taken in DMSO at a concentration of 100 mg/ml as the extract of honey for further studies.
The extracts were prepared using the solvents such as methanol, ethanol and ethyl acetate. All the extracts dissolved in DMSO were collected in sterilized glass tube and used within 24 h for the evaluation of bacteriostatic and bactericidal activity. After extraction residue of each honey were also checked for their antibacterial activity. The complete extraction plan can be better understood from flow diagram (Figures 1 to 5).
Inoculum Preparation
The bacterial slants were incubated overnight at 37ºC. 0.5 McFarland density of bacterial culture were adjusted in normal saline (85%) using densitometer to achieve the final concentration 1X 108 cfu/ml of each test organism individually. This had been used as adjusted inoculum for all the further studies.
Agar well diffusion assay (Zone of Inhibition Evaluation)
Antibacterial activity of all the extracts were evaluated by zone of inhibition using the agar well diffusion assay [13-15]. 100μl of each of the adjusted cultures were mixed into separate 100 ml of sterile, molten, cool MHA, mixed well and poured into sterile petri plates. These were allowed to solidify and then individual plates were marked for the organism inoculated. Each plate was punched to make 4 wells of 6 mm diameter with the help of a sterile cork borer at different sites of the plates.100 μl of respective solvents extracts and solvents (used as controls) were pipetted into the wells in assay plates. Plates were incubated overnight at 37ºC. Inhibition zones were observed, the diameter of which measured by using a Vernier caliper.
Determination of Minimum Inhibitory Concentration (MIC)
The minimum inhibitory concentration (MIC) of active extract was evaluated by tube dilution method. The MIC of all the extract was determined by dilution of the extract to various concentrations (5.000 to 0.150 mg/mL) as NCCLS [16]. Decreasing concentrations of methanol extract were prepared in serial twofold dilutions using Soybean casein digest broth (SCDB). 100μl standard inoculum of the microorganism (0.5 McFarland) was added to an equal volume (5ml) of each concentration and to a tube of the growth medium without methanol extract that served as growth control. An uninoculated tube of SCDB was incubated to serve as a negative growth control. In one tube methanol was added to SCDB which served as solvent control. After overnight incubation at 37º C, the tubes were examined for turbidity indicating growth of the microorganisms. The lowest solution of the extract that inhibited growth of the microorganism as detected by the lack of visual turbidity (matching the negative growth control) was designated the minimum inhibitory concentration.
Determination of Minimum Bactericidal Concentration (MBC)
The bactericidal activity of the extract was tested as follows: the number of the bacteria in the initial microorganism suspension was counted by the surface plate method [17]. After ascertaining the MIC, the number of bacteria was counted in each of the tubes of broth that showed no visible turbidity after overnight incubation, and was compared with the number of bacteria in the initial microorganism suspension. According to NCCLS, the lowest concentration of the extract solution that allowed less than 0.1% of the original inoculum to survive was taken to be the minimum bactericidal concentration.
Results and Discussion
All the extracts of honey samples exhibited varying level of antibacterial activity against all the selected strains as indicating by the zone of inhibition of growth (Table 1).
| Extracts | Zone of inhibition* | |||||
|---|---|---|---|---|---|---|
| Gram-positive bacteria | Gram-negative bacteria | |||||
| RH-1 | S. aureus | B. subtilis | B. cereus | P. aeruginosa | E. coli | S. typhi |
| Ethanol | 8.90 | 8.90 | 12.83 | 32.35 | 17.51 | 31.85 |
| Methanol | NZ | 8.55 | 11.11 | 35.95 | 26.49 | 34.39 |
| Ethyl acetate | 9.51 | 11.19 | 11.46 | 13.09 | 17.15 | NZ |
| Residue | NZ | NZ | NZ | NZ | NZ | NZ |
| RH-2 | ||||||
| Ethanol | NZ | NZ | NZ | 25.06 | 17.28 | 29.47 |
| Methanol | 10.0 | NZ | NZ | 26.00 | 25.41 | 31.18 |
| Ethyl acetate | NZ | NZ | NZ | NZ | NZ | NZ |
| Residue | NZ | NZ | NZ | NZ | NZ | NZ |
| PH-1 | ||||||
| Ethanol | NZ | 7.83 | 6.94 | 23.43 | 16.14 | 35.92 |
| Methanol | 9.64 | NZ | NZ | 33.40 | 28.49 | 37.94 |
| Ethyl acetate | 9.15 | 7.25 | NZ | 24.60 | 17.75 | 36.58 |
| Residue | NZ | NZ | NZ | NZ | NZ | NZ |
| PH-2 | ||||||
| Ethanol | NZ | NZ | NZ | 21.22 | 14.00 | 30.12 |
| Methanol | 8.90 | 9.72 | 10.02 | 31.22 | 29.40 | 38.12 |
| Ethyl acetate | NZ | NZ | NZ | 22.44 | 19.72 | 34.40 |
| Residue | NZ | NZ | NZ | NZ | NZ | NZ |
| Antibiotics (Positive control) | ||||||
| Tetracycline | 26.44 | 21.01 | 17.01 | NZ | 16.03 | 26.96 |
| Ciprofloxacin | 14.75 | 19.01 | 16.67 | 19.01 | 16.67 | 14.75 |
| Solvents (Negative control) | ||||||
| Ethanol | NZ | NZ | NZ | NZ | NZ | NZ |
| Methanol | NZ | NZ | NZ | NZ | NZ | NZ |
| Ethyl acetate | NZ | NZ | NZ | NZ | NZ | NZ |
| DMSO | NZ | NZ | NZ | NZ | NZ | NZ |
Table 1. In vitro antibacterial activity of Honey (Apis mellifera).
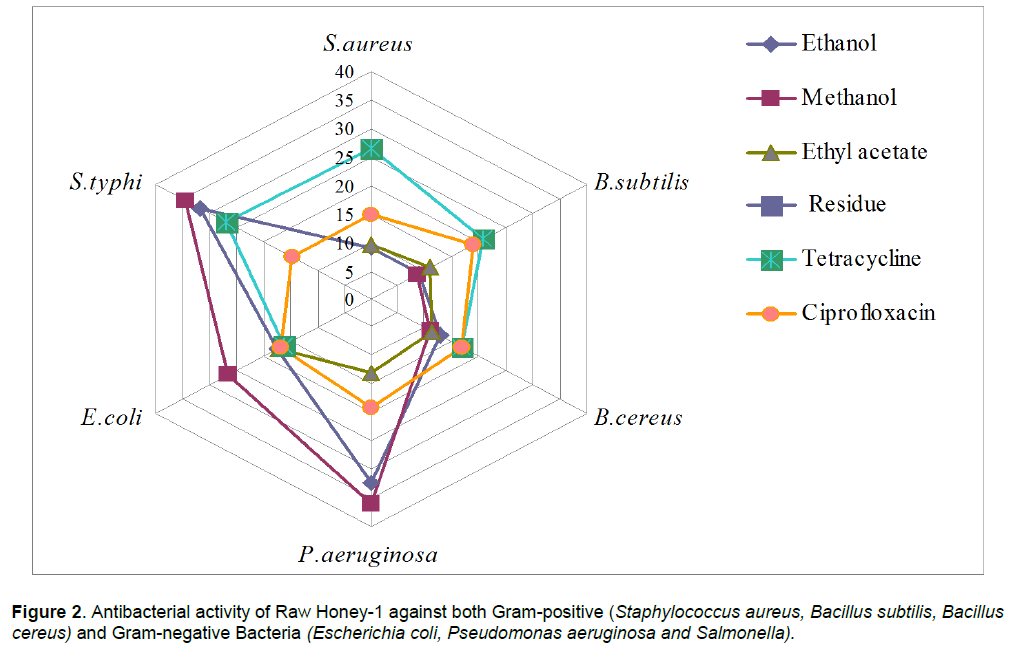
In case of raw honey-1, the maximum inhibition as produced by extracts was observed against P. aeruginosa (35.95 mm zone size)< S. typhi (34.39 mm zone size) <E. coli (17.51 mm zone size)< B. cereus (11.11 mm zone size) < S. aureus (8.90 mm zone size). < B. subtilis (8.55 mm zone size). However methanol extract and ethyl acetate extract was found to be inactive as no zone of inhibition observed against S. aureus and S. typhi respectively. Residue obtained after extraction was also found inactive against all the tested strains.
Ibrahim [18] and Jeddar et al. [19] reported the bactericidal activity of aqueous solution of honey on Salmonella spp. and Shigella spp. as also enteropathogenens such as E. coli, Vibrio cholerae and other Gram-negative and Gram-positive bacteria. Similarly, Allen et al. [20] reported the antibacterial properties of honey against two laboratory isolates e.g. P. aeruginosa and E.coli.
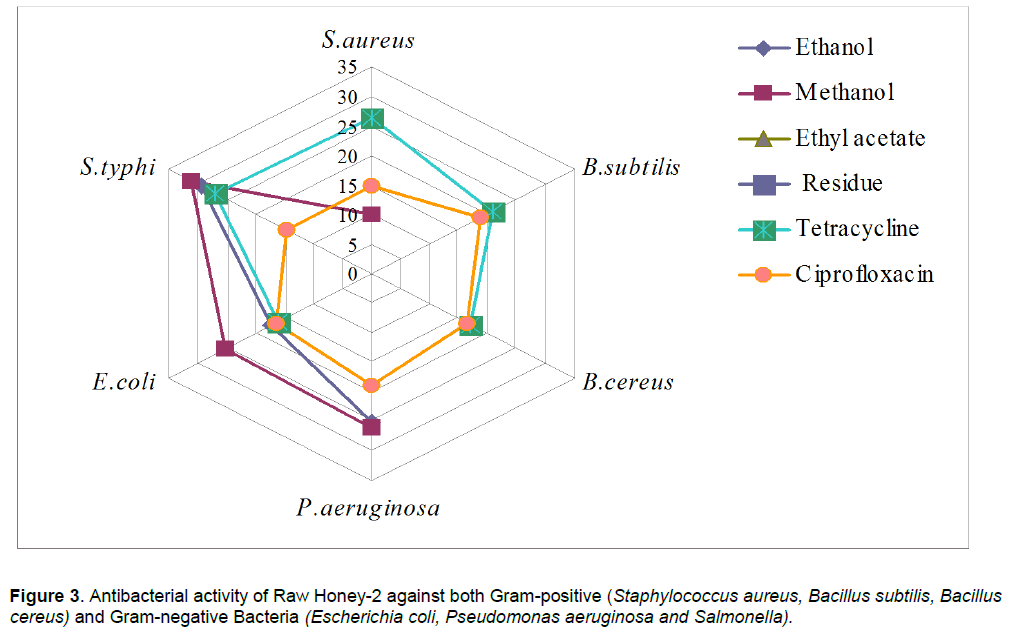
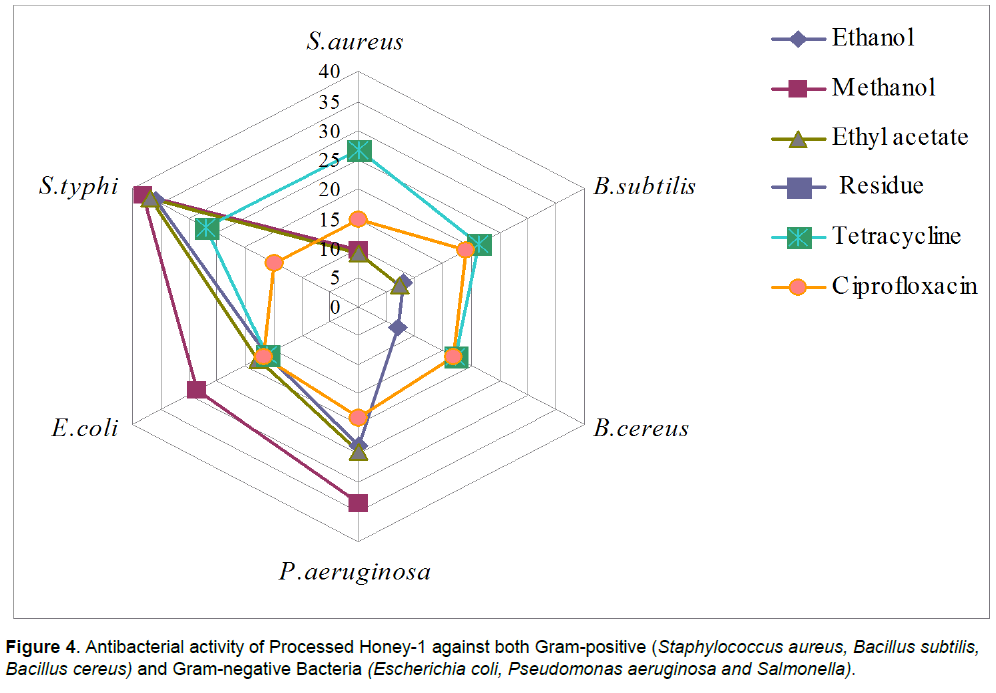
In case of raw honey-2, the maximum inhibition as produced by extracts was observed against S. typhi (31.18 mm zone size)> P. aeruginosa (26.00 mm zone size) <E. coli (25.41 mm zone size < S. aureus (10.00 mm zone size). Ethyl acetate extract was found completely inactive as no zone of inhibition observed, however ethanol extract was found inhibitory only against Gram-negative bacteria. B. subtilis and B. cereus showed resistance to methanol extract. Residue obtained after extraction was also found inactive against all the tested strains. In case of processed honey-1, the maximum inhibition as produced by extracts was observed against S. typhi (37.94 mm zone size)> P. aeruginosa (33.40 mm zone size) E.coli (28.49 mm zone size) and little zone of inhibition was observed against gram-positive bacterial strains. However, all extracts were found inactive against one or more than one organisms, ethanol extracts against S. aureus, methanol extracts against B. subtilis and B. cereus and ethyl acetate extracts against B. cereus only. Residue obtained after extraction was also found inactive against all the tested strains.
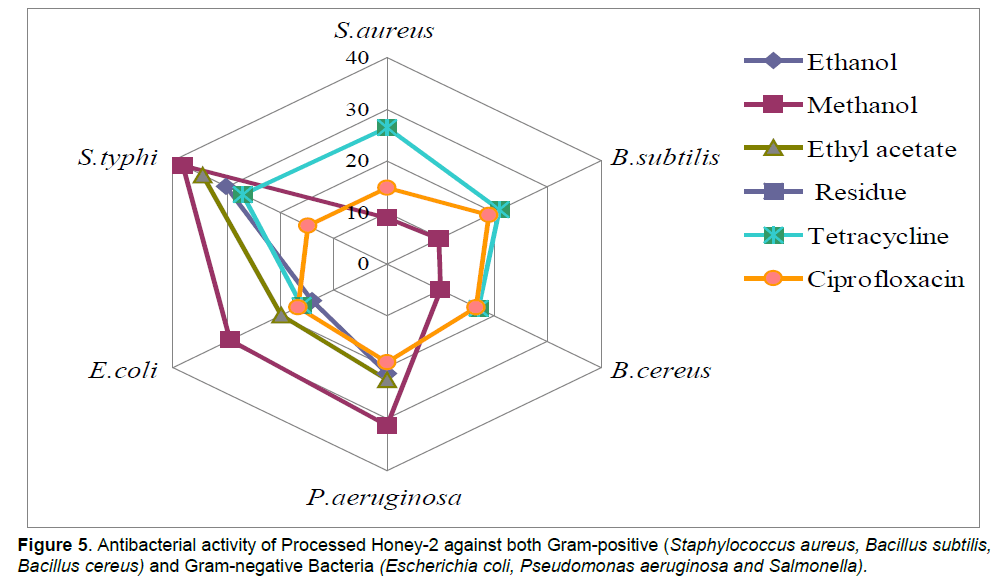
In case of processed honey-2, the maximum inhibition as produced by extracts was observed against S. typhi (38.12 mm zone size)> P. aeruginosa (31.22 mm zone size) <E. coli (29.40 mm zone size). Ethanol and ethyl acetate extracts were found inactive against Gram-positive bacteria. Standard antibiotics (Ciprofloxacin and tetracycline) were also taken to check the activity against all the bacterial strains. Gram-negative bacteria were found more susceptible then Gram-positive bacteria.
Adeleke et al. [8] has also reported the strong inhibitory properties of honey against a total number of fifty isolates of P. aeruginosa and E. coli from various pathologic sources.The extracts obtained with zone of inhibition more than 15 mm were subjected to macrodilution assay to determine the MIC as shown in Table 2.
| Extracts | P. aeruginosa | E. coli | S. typhi | |||
|---|---|---|---|---|---|---|
| RH-1 | MIC (mg/ml) | MBC (mg/ml) | MIC ((mg/ml) | MBC (mg/ml) | MIC (mg/ml) | MBC (mg/ml) |
| Ethanol | 1.250 | 2.500 | 2.500 | 5.000 | 1.250 | 2.500 |
| Methanol | 0.625 | 1.250 | 1.250 | 1.250 | 0.625 | 0.625 |
| Ethyl acetate | 2.500 | 5.000 | >5.000 | >5.000 | >5.000 | >5.000 |
| RH-2 | ||||||
| Ethanol | 1.250 | 2.500 | 2.500 | 5.000 | 1.250 | 2.500 |
| Methanol | 0.625 | 1.250 | 0.625 | 1.250 | 0.625 | 0.625 |
| Ethyl acetate | ND | ND | ND | ND | ND | ND |
| PH-1 | ||||||
| Ethanol | 1.250 | 1.250 | 2.500 | 2.500 | 1.250 | 1.250 |
| Methanol | 0.625 | 0.625 | 0.625 | 1.250 | 0.625 | 0.625 |
| Ethyl acetate | >5.000 | >5.000 | >5.000 | >5.000 | 2.500 | 2.500 |
| PH-1 | ||||||
| Ethanol | 1.250 | 2.500 | 2.500 | 2.500 | 1.250 | 1.250 |
| Methanol | 0.625 | 1.250 | 0.625 | 1.250 | 0.625 | 0.625 |
| Ethyl acetate | >5.000 | >5.000 | >5.000 | >5.000 | 1.250 | 1.250 |
| Antibiotics (Positive control) | ||||||
| Tetracycline | ND | ND | <0.150 | <0.150 | <0.150 | <0.150 |
| Ciprofloxacin | <0.150 | <0.150 | <0.150 | <0.150 | <0.150 | <0.150 |
Table 2. MIC and MBC values of different extracts of Honey (Apis mellifera).
In case raw honey 1, the lower MIC and MBC value was observed against S. typhi (0.625 mg/ml) however it was found to be more than 5.000 mg/ml against E. coli as no growth observed in the tube. In case raw honey 2, MIC and MBC value was observed in the range of 0.625 mg/ml to 5.000 mg/ml. In case of processed honey 1 and 2, lower MIC and MBC value of methanol extract was observed against all the gram-negative bacteria. However, MIC and MBC values of ethyl acetate extract was found to be more than 5.000 mg/ml against P. aeruginosa and E. coli. MIC and MBC values of standard antibiotics were found less than 0.15 mg/ml.
Antibacterial activity of honey may be because of the ability of honey to kill microorganisms has been attributed to its high osmotic effect, high acidic nature (pH being 3.2-4.5), hydrogen peroxide concentration and its phytochemical nature, i.e. its content of tetracycline derivatives, peroxides, amylase, fatty acids, phenols, ascorbic acid, flavonides, streptomycin, sulfathiazole, trepens, benzyl alcohol and benzoic acids [10,22,23].
Similar studies have been reported in the form of antibacterial activity of honey against Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, P. mirabilis, Streptococcus pyogenus, S. flexneri and Salmonella typhi [10,24,25]. The in vitro antimicrobial activity of honey was also reported by Radwan et al. [26] who observed that honey stopped the growth of Salmonella and Escherichia coli.
Conclusions
Following conclusion can be drawn from the present study:Honey both raw as well as processed can be the potential antimicrobial substance for control of different types of bacterial pathogens.
1) It was found that honey exhibited both bacteriostatic and bactericidal properties on both Gram-positive as well as on Gram-negative bacteria.
2) Residue left after extraction with various solvents exhibited almost negligible antibacterial activity.
3) The antibacterial activity of all the solvent extract was found to be significant against Gram-negative pathogens.
4) The solvent extracts of honey were found to be bactericidal against P. aeruginosa for which even commonly used synthetic antibiotic such as tetracycline was found ineffective.
5) Chemical antibiotics have been become absolete within a short period of time. The need for an alternative antibacterial substance derived from natural materials on a sustaninabe manner has become a subject of interest for the scientists worldover. The result of this study clearly shows that the extracts of honey in ethanol, methanol and ethylacetate solvents can become a potential candidate as an antibiotic, which would be useful on a sustainable basis.
6) This study shows that the other bulk components can be utilized elsewhere, while the necessary component is extracted from honey for antibacterial purposes. If found successful, it will pave way for developments leading to the exploitation of honey extracts for antibiotic activity as well as for other conventional uses of honey (bulk components).
7) The bactericidal and bacteriostatic effect of honey reported here has the potential for health care in rural areas once the pharmacological standardization and clinical trials are done, honey would become the house hold product not just for cure but even for preventive cure.
8) Further this study would pave the way to the studies more related to raw and processed honey of different types to understand the antimicrobial properties with special reference to resistant microbial strains.
References
- World Health Organization. (1999) Drug information. WHO, Geneva, 13(4): 230-233.
- Assefa A., Yohannes G. (1997) Antibiotic sensitivity of S. aureus and E. coli strains isolated in Gondar, Ethiopia. Tropical Doctor, 27(2): 121-126.
- Shears P. (2000) Antimicrobial resistance in the tropics. Tropical Doctor, 30(2): 114-116.
- Molan P.C., Smith I.M., Reid G.M., et al. (1988) A comparison of the antibacterial activities of some. Journal of Agricultural Research, 27: 252–256.
- Ali A.T., Chowdhury M.N., Al-Humayyd, M.S., et al. (1991) Inhibitory effects of natural honey on Helicobacter pylori. Tropical gastroenterology, 12: 139- 143.
- Krell R. (1996) Value-added products from Bee keeping. FAO agricultural.
- Lusby P.E. Coombes A., Wilkinson J. M., et al. (2002) Honey: A potent agent for wound healing? J. Wound Ostomy Continence Nurs, 29: 295-300.
- Adeleke O.E., Olaitan J.O., Okepekpe E.I., et al. (2006) Comparative antibacterial activity of honey and Gentamicin against Escherichia coli and P. aeruginosa. Annals of burn and fire disasters, 19: n4.
- Basualdo C., Sgroy V., Finola M.S., Juam M., et al. (2007) Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Veterinary Microbiology, 124(3-4): 375-381.
- Molan P.C. (1992) The antibacterial activity of honey. Bee world, 73(1): 5-28.
- Namias S. (2003) Honey in the management of infections. Surg. Infect (Larchmt), 4: 219-226.
- Farouk H.F., Kash S.A., Khalid M., Mahasin W., et al. (1988) Studies on sudanese bee honey. Int. J. Crude Drug Res, 26: 161-168.
- Perez C., Pauli M., Bazerque P., et al. (1990) An antibiotic assay by the agar-well diffusion method. Acta Biologiae at Medecine Experimentalis, 15: 113-115.
- Goyal P., Chauhan A., Kaushik P., et al. (2009) Laboratory evaluation of crude extracts of Cinnamomum tamala for potantial antibacterial activity. Electronic Journal of Biology, 5(4): 75-79.
- Kaushik P., Goyal P., Chauhan A., Chauhan G., et al. (2009) In vitro evaluation of antibacterial potential of dry fruit extracts of Elettaria cardamomum Maton (Chhoti Elaichi). Iranian J Pharmaceutical Research, 9(3).
- NCCLS. (1997) National Committee for Clinical Laboratory Standards. Methods for dilution antimicobial susceptibility tests for bacteria that grows aerobically. Approved Standards, M7-A4, Wayne, Pa.
- Collins C. H. (1984) Microbiological methods. Butter worth & Co (Publisher) Ltd. pp. 1-448.
- Ibrahim A. S. (1985) Antibacterial action of honey. Bull. Islam Med, 1: 363-365.
- Jeddar A., Kharsany A., Ramsaroop U. G., Bhamjee A., Hafejee I. E., Moosa A., et al. (1985) The antimicrobial action of honey. South Afr. Med. J, 67: 257-258.
- Allen K. I., Radwan S., Reid G. M., et al. (2000) Antimicrobial activity of honey on some microbial isolates. J. Medical and Pharmaceutical Sciences, 2: 75-79.
- Molan P.C., Cooper R.A. (2000) Why honey and sugar as dressing for wound and ulcer. Trop. Doc., 4: 249- 258.
- Bogdanov S. (1984) Characterization of antibacterial substance in honey. Lebensm Wiss Technol, 17(2): 74- 76.
- eerng W. (1998) Immunochemical screening for antimicrobial drug residue in commercial honey, 123(12): 2759-2762.
- Nzeako and Hamdi (2000) Antimicrobial potential of Honey. Medical Sciences, 2: 75-79.
- Kingsley A. (2001) Supplements. The use of Honey in treatment of infected wounds. Case studies. BJ of Nursing, 10(22): Sup 13-Sup 20.
- Radwan S. A., El-Essawy, Sarhan M. M. et al. (1984) Experimental evidence for the occurrence in honey of specific substances active against microorganisms. Zentral Mikrobiol, 39: 249-255.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences