An Approach to a Patient of Amenorrhea - Case Report and Review of Literature on Premature Ovarian Failure (POF)
Kulvinder Kochar Kaur, Gautam Allahbadia, Mandeep Singh
Kulvinder Kochar Kaur1,*, Gautam Allahbadia2 and Mandeep Singh3
1 Centre for Human Reproduction, Jalandhar, Punjab, India;
2 Department of Obstetrics and Gynecology, D.N.B, Rotunda-A Centre for Human Reproduction, Bandra, Mumbai, India
3 Swami Satyanand Hospital, Near Nawi Kachehri, Jalandhar, Punjab, India.
Received date: September 15, 2016; Accepted date: October 31, 2016; Published date: November 07, 2016
Citation: Kaur KK, Allahbadia G, Singh M. An Approach to a Patient of Amenorrhea - Case Report and Review of Literature on Premature Ovarian Failure (POF). Electronic J Biol, 12:4
1. Introduction
Primary Amenorrhea (PA) is defined when a patient does not get periods by the age of 14 in the absence of secondary sex characters or ii) if there are no periods by age of 16 irrespective of secondary sex characters having got developed. iii) Secondary Amenorrhea (SA) is the diagnosis in a woman who had been menstruating earlier ;if she has absence of periods for a length of time equivalent to a total of at least 3 of the previous cycle intervals or 6 months of amenorrhea, a diagnosis of amenorrhea is made and she needs to be evaluated. A full thorough examination of secondary sex characters like breast development and axillary and pubic hair along with tanner staging is important in primary amenorrhea, besides presence of a hymen which is patent and a vagina. If patient allows presence of uterus is confirmed by per rectal examination, otherwise it is confirmed by Ultrasonography (USG).
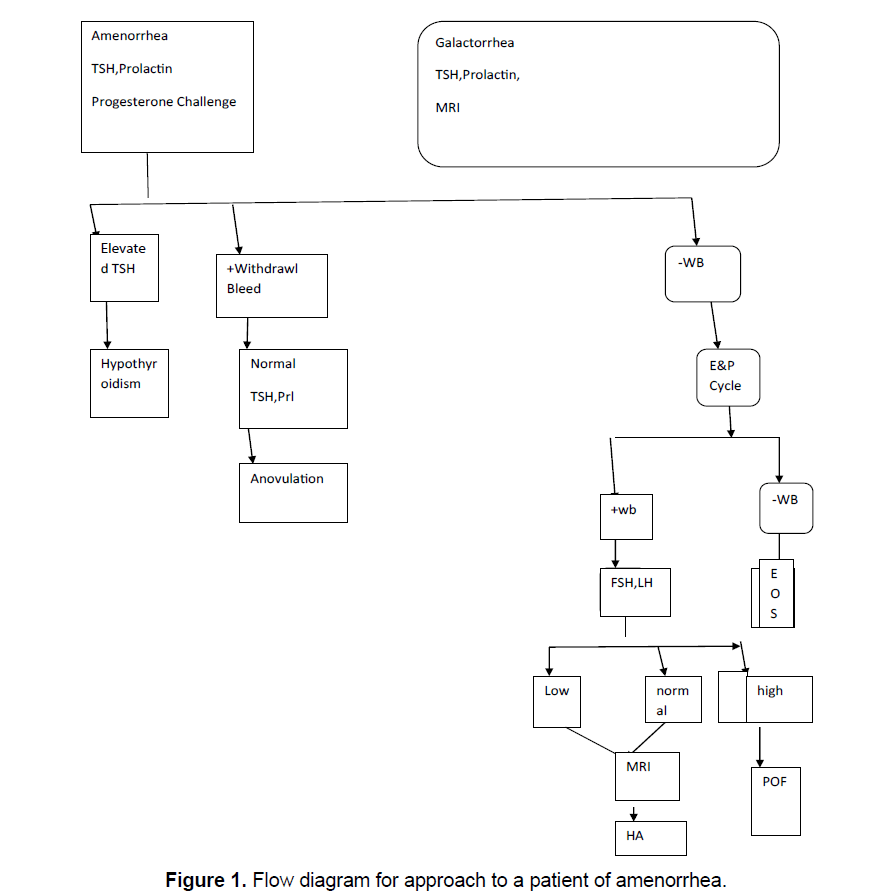
Detailed history and physical examination including evidence of psychogenic dysfunction or emotional stress, family history of apparent genetic anomalies with a focus on nutritional status, abnormal growth and development, the presence of a normal reproductive tract and examination is followed by step by step investigations, following exclusion of pregnancy, starting with serum TSH, Prolactin levels and a progesterone challenge as well as the evidence of a CNS disease. If galactorrhea accompanies amenorrhea, then sellar imaging is included, see Figure 1 to see how to approach a patient of amenorrhea.
The duration of hypothyroidism is important with respect to mechanism of galactorrhea; longer the duration, greater is the incidence of galactorrhea and greater the prolactin levels [1]. This is believed to be due to decreasing hypothalamic dopamine content with ongoing hypothyroidism. This leads to an unopposed TRH stimulatory effect on the pituitary cells that secrete prolactin. Usually prolactin levels associated with hypothyroidism are <100 ng/ml. Once there is a constant stimulation of hypothalamic releasing hormones; it leads to hypertrophy or hyperplasia of the pituitary. Thus, the imaging picture of a tumor be it distortion, expansion or erosion of the sella tursica can be seen. Hence in primary hypothyroidism as well as in patients with elevated GnRH and gonadotropin secretion due to P.O.F., imaging studies in the form of computed tomography (CT scan or Magnetic Resonance Imaging (MRI) is indicated [2,3]. Proper treatment is followed by rapid normalization of the initial picture. Patients with primary hypothyroidism and hyperprolactinemia can either present with primary or with secondary amenorrhea [4].
Next a progesterone challenge test is done to see the endogenous estrogen along with the competence of the outflow tract. A progestational agent course totally devoid of estrogenic activity is given. It can be given either parenterally as Progesterone (P) in oil in a dose of 200 mg or orally as micronized P, 300 mg/d or as orally active medroxy progesterone acetate, in a dose of 10 mg/d for 5 days.Oral administration avoids the unpleasant im injection (only necessary if compliance is a problem). Within 2-7 days of stoppage of P the patient will bleed or not bleed. If bleeding occurs a diagnosis of anovulation is reliably established, along with the presence of a functional outflow tract and a uterus lined by reactive endometrium. Once the presence of estrogen is demonstrated, minimal function of ovary, pituitary, and CNS gets established.
If at any time a patient fails to have withdrawal bleed in absence of pregnancy it means patient has shifted to the next category of negative withdrawal bleed, and further follow-up is required. Occasionally progestational challenge will trigger an ovulationthe clue will be withdrawl bleed after 14 days of the progestational challenge. In the absence of galactorrhea and a normal serum prolactin, nothing further has to be done, except treating the anovulaory status Reviewed in reference [5].
1.1 Step 2
If there is a negative P withdrawl bleed that means the target outflow tract is inoperative or that the preliminary Estrogen (E2) proliferation of the endometrium has not occurred. To clarify this orally active E2 is administered in the form of conjugated estrogen1.25 mg/estradiol valerate 2 mg, for 21 days. Terminally oral medroxy P acetate is given as 10 mg/d for 5 days, which is essential to achieve withdrawal bleed. In case of absence of withdrawal bleed a precautionary 2nd dose is given. By this the compartment 1 gets challenged by exogenous E2. With this the amenorrheic patient will bleed or not. With no withdrawal bleed a defect in compartment 1 can be made with confidence. If bleeding occurs that means the compartment 1 is normal. Practically in patients with normal external and internal genitalia on pelvic examination and absence of history of infection or trauma (e.g. curettage) an abnormality of the outflow tract is not likely. Abnormalities of the compartment 1 are not commonly encountered and in absence of reason to suspect one can omit step 2.
1.2 Step 3
If an amenorrheic patient is unable to produce adequate E2, the physiological mechanism for steroid elaboration needs to be tested. To produce E2, ovaries containing a normal follicular apparatus, besides sufficient gonadotropins to stimulate this apparatus are needed. Step 3 is to test where there is malfunction in these 2 components. In this gonadotropin assay is required. Since in step 2 exogenous E2 is given step 3 should be delayed 2 weeks from step 2. A midcycle LH surge is 3 times normal hence if no withdrawal bleed occurs 2weeks following that then that LH level is abnormal. The gonadotrophin levels will be abnormally high (FSH>20 iu/l, LH>40 iu/l, abnormally low (Both FSH and LH<5 iu/l) or within normal range (5-20 iu/l), in those not getting a progesterone withdrawal bleed.
2. Premature Ovarian Failure (P.O.F)
The early depletion of follicles is surprisingly common. Roughly 1% women will experience P.O.F before the age of40, while in women with primary amenorrhea the prevalence ranges from 10%-28% [6-8].
The etiology of P.O.F is unknown in most case. But it is better to explain to the patient that it is possibly a genetic disorder with an increased risk of follicle disappearance. Specific sex anomalies can be identified often [9]. Commonest ones are 45X and 47XXY, followed by mosaicism and specific structural abnormalities on the sex chromosomes. On searching for 45X/46XX mosaicism using fluorescent in situ hybridization, a larger percentage of cells containing single X can be detected in women who present with P.O.F [10]. Translocations in the critical region on the long arm of chromosome have been described in women with P.O.F [11,12]. Mechanism of ovarian failure is mostly due to accelerated follicular atresia as even in TS, patients begin with a full follicle complement of germ cells. P.O.F is more common in families which contain the fragile X syndrome, which is a relatively common cause of developmental disability, which suggests that it would be useful to screen for fragile X syndrome in families with a history of P.O.F [13]. It is important to understand that carriers of fragile X syndrome are at an increased risk of P.O.F. but no other medical problem [14]. Blepharophimosis/ptosis/epicanthus inversus syndrome which is an autosomal dominant condition has been associated with eyelid abnormality and P.O.F, which is caused by mutations in a transcription factor gene FOXL2 on chromosome 3 [15]. Besides that P.O.F, can be due to an autoimmune process or perhaps destruction of follicles by infections like mumps, oophoritis or a physical insult like irradiation or radiotherapy.
The problem can present at varying ages on the basis of number of follicles left. If loss of follicles is rapid then primary amenorrhea and lack of sexual development will be present. In case loss of follicles occurs after puberty, then the extent of adult phenotypic development and time of onset of secondary amenorrhea will vary accordingly. Although many cases resume spontaneous menses with a normal karyotype this does not warrant a full thickness ovarian biopsy. Minimal approach, i.e., recommended, with no definitive method to rule out autoimmune disease and checking the O-P activity. As far as hormonal therapy is concerned in view of hypogonadal patients in view of spontaneous ovulations with E-P contraceptive therapy, that is the treatment of choice. Best chance of pregnancy is with donor oocytes; important to note that with sibling’s oocytes pregnancy rates are reduced [16]. In idiopathic P.O.F treatment with corticosteroids is not warranted as responsiveness to gonadotropins is not achieved [17].
2.1 Molecular explanation for ovarian failure
In a group of patients normal chromosome pattern has been identified with P.O.F in Finland displaying an recessive inheritance pattern [18]. In this population a point mutation of FSH Receptor was demonstrated to be the cause of ovarian failure in this population [19]. It accounted for 29% of 75 Finnish women presenting with P.O.F, with a general prevalence of 0.96 [20]. Ovarian follicles were present in these ovaries although ovaries were small on USG. A large number of inherited conditions is present in Finland. Trying to search the same mutation in the US, Brazil, Switzerland, Denmark, Japan and Singapore could detect only a single case in Switzerland in patients presenting with P.O.F [21-23]. In Finland, other specific mutations in the FSH receptor gene have been identified, but they remain very rare causes of P.O.F [24]. However it should be expected that an occasional patient of P.O.F will have mutation of the FSH receptor gene [25-27]. The management does not get much affected by documenting a specific mutation or genetic cause of hypogonadism.
As more and more patients are investigated for genetic studies, multiple subgroups, stand to be identified, each with a different biological cause of ovarian failure, e.g. a case of hypergonadotropic primary amenorrhea has been reported due to point mutation in the LH receptor gene; FSH and LH were only mildly elevated with multiple ovarian follicles present with development and steroidogenesis upto early antral stage [28]. The reason gonadotropins are almost within normal range is due to inhibition by inhibin, as inhibin secretion by granulosa cells is FSH dependent, not being influenced by LH. This same patient had two siblings who were 46XY male pseudohermaphroditism due to Leydig cell hypoplasia because of same LH receptor mutation. In another example translocations on regions on X and Y chromosome which share sequence homology, have been reported with ovarian failure [29]. Sequences on the long arm of X chromosome (Xq27-28) share homology with long arm of the Y chromosome (Yq11.22) allowing errors in the process of crossing over. Besides that, less than complete mutations also known as permutations of the site that transmits the fragile X syndrome have been reported to occur in a greater frequency in women with P.O.F [30]. Deletions of the X chromosome are rare in secondary amenorrhea, but occasionally a deletion can be detected in women with a familial history of P.O.F [31].
3. Case Report
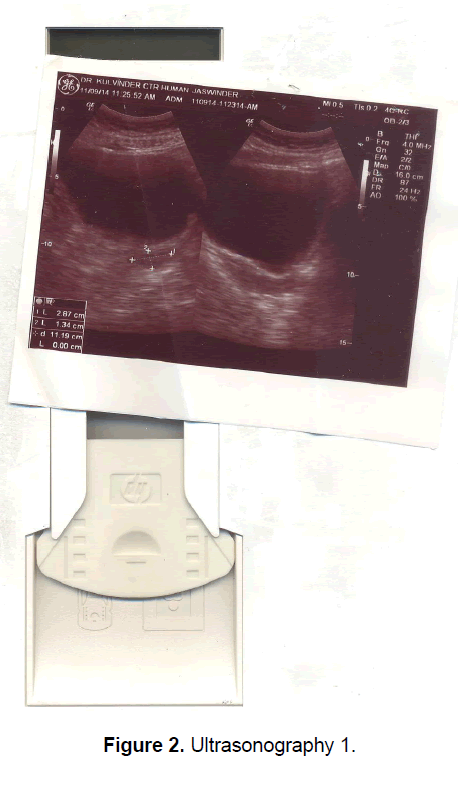
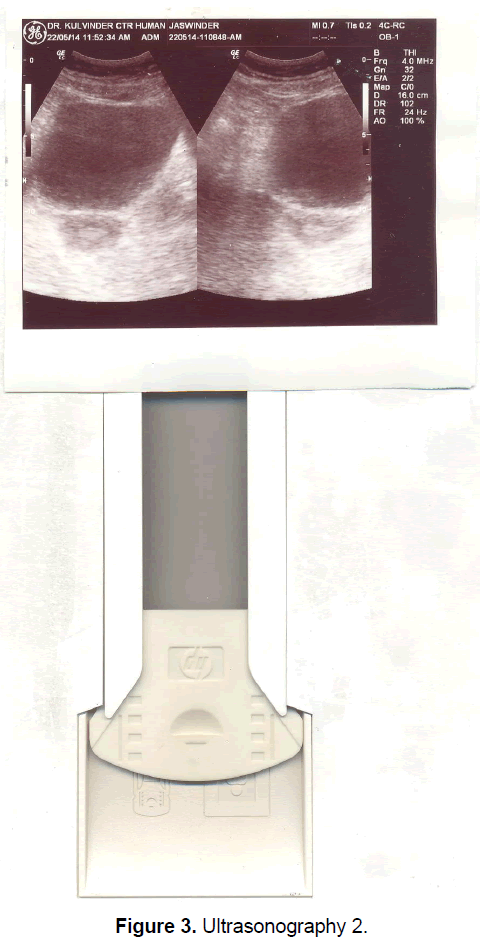
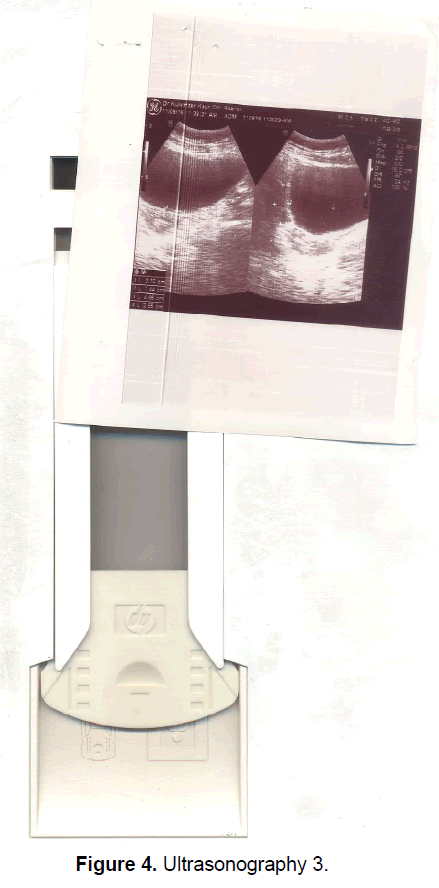
A 21 year old unmarried girl presented with primary amenorrhea. She gave a history of diminution of vision both near and distant for the past four years, which was ultimately diagnosed to be retinitis pigmentosa. Mother gave history of taking some abortifacient during pregnancy (nature?) although other than threatened abortion, pregnancy continued till term and she delivered vaginally. On examination she was average built with wt 74 kg, ht- 166 cm, BMI- 26.5 Kg/m2.On examination she had well developed breasts, tanner stage IV, with no galactorrhea, along with well-developed axillary and pubic hair, tanner stage IV. On local examination hymen was intact with a normal developed vagina. Per rectum examination revealed a small nodular uterus. Ultrasonography showed a small hypoplastic uterus measuring 26 × 24 × 13.9 mm and both ovaries were difficult to visualize although right ovary was somewhat visualized, being quiet – atretic (Figures 2-4). BP was 110/70 mm hg, on investigations serum FSH and LH were raised, FSH-77 iu/L, LH-41.26 iu/L. Repeat test confirmed values of 79 and 45 iu/L respectively for FSH and LH, S Prolactin-19.4 ng/dl (2.4-29 ng/dl), Thyroid function tests were within normal limits. Karyotyping was 46XX, S.AMH-0.78(0.3-2.2 ng/ml), Rheumatoid Factor (RF) - 22.35 (<30 IU/ML), antinuclear antibody (ANA)-0.55 (<1.0 s) MRI Brain was done to rule out any neoplasm in CNS with the visual defect but no MRI was done for pelvic area.
After a course of ethinyl estradiol 50 μg and medroxy progesterone acetate in latter 10 days for 3 cycles her uterus size increased to 46.6 × 36 × 23.3 cm. She also started getting a good withdrawal bleed Despite trying a prophylactic wysolone no benefit was observed. Then a course of dehydrepiandroserone sulfate (DHES) was tried for 3 months when her ovaries became more prominently visible and S FSH -23.3 and LH-7.33 IU/L although a further course has been tried to see if one can reverse this.
4. Discussion
As discussed earlier incidence of P.O.F is 10-28% in primary amenorrhea with etiology difficult to pinpoint in patients having normal karyotype although genetic etiology is suggested. Recently point mutations of FSH receptor gene were found in Finnish women. Similar point mutations have been found occasionally in LH receptor gene, but since these do not affect the treatment and patient cannot afford a test of over Rs. 30, 000 we did not go for the same. Right now her priority is her vision for which although currently no method is recommended worldwide, we are exploring if somehow by stem cell technology she may benefit. Right now we are pursuing with another course of DHES to somehow be able to retrieve some cohort of primordial follicles if possible otherwise it leaves her with no option but for donor egg IVF once she opts for marriage and fertility. In view of her normal rheumatoid factor and antinuclear antibody one did not expect an autoimmune aetiology as has been seen in 4-30% of POI patients and even prophylactic wysolone had no impact [32]. The reason why she had a rudimentary uterus was because POF set in before the onset of puberty unlike the patients having normal karyotype and in turners/mosaics it is uninhibited meiotic division which led to an atretic ovary. Once she received exogenous ethinyl estradiolfor 3 weeks just like the endogenous hormonal milieu when a patient is developing a follicle and has normal endogenous hormonal milieu her myometrium as well as endometrium proliferated because of which her uterus size increased to 46.6 × 36.6 × 23.3 and addition of progesterone gave a normal maturation of endometrium because of which there was a proper withdrawal bleed once these hormones were withdrawn. In the study of Qin, based on searching PubMed and Google scholar articles till may 2015 chromosomal abnormalities like monosomy, mosaicism, X deletions and rearrangement of X, autosomal translocations were identified in 10-13%. Besides the chromosomal abnormalities, Qin et al. identified the candidate genes which unequivocally affected at least one population included bone morphogenetic protein 15 (BMP15), Progesterone receptor membrane component 1 (PRMC1), Fragile X Mental Retardation1 (FMR1), Growth differentiation factor 9 (GDF9), Folliculogenesis specific bHLH transcription factor (FIGLA). Newborn ovary homeobox gene (NOBOX), Nuclear receptor subfamily5, group A, member1 (NR5A1) Nanos homolog 3(NANOS3) also were seen in 1-2% of single population studied. Whole genome approaches used genome wide association studies (GWAS) to show loci, which were not predicted on basis of a causative gene but remained difficult to locate, although not always replicated, but it remained difficult to locate candidate genes and susceptible loci were not always replicated. Cytogenetic methods (array cGH) have identified other regions of interest but studies have not shown consistent results, the resolution of array has varied and replicating is uncommon .Whole exon sequencing in non-syndromic POI, has only begun recently-mutations in the stromal antigen 3 (STAG3), synaptonemal complex central element 3 (SYCE1), mini chromosome maintenance complex competence 8&9 (MCM8, MCM9) and ATP dependent DNA helicase homolog (HFMD gene).Thus they concluded together cytogenetic, cytogenetic (array cGH)and exon sequencing approaches has shown 20-25% of POI cases showing a genetic cause. Thus the remaining genes or causative genes would be facilitated by not only by whole genomic approaches which involved long cohort in multiple populations but also incorporated environmental exposure and exploring signaling pathways in intragenic and intergenic regions which point to perturbations in regulatory gene networks [33].
Similarly Chapman et al. reviewed the genetic causes including oocyte specific transcription factors, like NR5A1, NOBOX like genes and, FIGLA and FOXL2 GENES and folliculogenesis growth factors like BMP15and GD F9 (both belonging to TGFβsuperfamily growth factors) [34].
Further various micro RNA’s like miR23afamily promotes granulosa cell apoptosis via repression of X linked inhibitor of apoptosis (XIAP) expression suggesting the differential expression of miR23a may be a potential candidate of POF development. Because of small sample size, the role of mir23in the etiology of POF is unclear [35]. More currently Dang et al investigated differential expression miR in a large cohort of Chinese women and found 22 significantly up regulated and 29 down regulated miR in 140 POF patients, as compared to controls. Among these mir22-3pwas significantly down regulated in POF and a negative association between serum micro22-3pand FSH was seen.
The researchers suggested miR22-3pmay regulate pituitary FSH secretion, as its expression has been seen in the pig pituitary whereby the decreased expression has been seen to contribute toward POF pathogenesis, Tsuilo, conducted a retrospective study on copy number variation analysis in 301 spontaneous POF patients with 3188 Controls from 2003-2014 at Estonian Genome Centre at University of Tartu Biobankand found 11 novel microdeletions which encompass genes which were relevant to POFeg [36]. FMN2 (Iq43) and SGOL (22q33.1) are essential for meiotic progression, while TBP (6q27), SCARB1 (12q24.31), BNC1 (15q25) and ARFGAP3 (22q13.2) are involved in follicular growth and oocyte maturation. They also corroborated the importance of recently discovered hemizygous microdeletions of meiotic genes, SYCE1 (10q26.3) and CPEB (15q25.2) in POF patients. The limitations were no functional analysis was done [37].
4. Conclusion
Thus as Chapman et al. [34] concluded that despite advanced knowledge of molecular basis and pathophysioloy of idiopathic POF is rapidly growing, unbiased approaches of GWAS and NGS techniques have highlighted the defects in folliculogenesis and DNA damage and repair pathways, as significant contributors to POF pathogenesis, providing basis for potential targeted treatment. Still it is unclear at present how to design these targets therapies to defects affecting molecular mechanisms like DNA damage and repair. Since POF has varied etiology one expects to see novel molecular pathways which might help us understand the regulation of ovarian function .Since very small number of genes affecting idiopathic POF, have been identified till date, more future studies need to be designed in large cohorts with unrelated POF patients to get the missing link of heritability in POF. Also in our patient since there is history of intake of some abortifacient relevance of alteration of epigenetics in view of potential role of miRNAs in FSH control has been seen also needs to be taken into consideration.
References
- Countreras P, Generini G, Michelson H, et al. (1981). Hyperprolactinaemia and galactorrhoea: Spontaneous versus iatrogenic hypothyroidism. J Clin Endocrinol Metab. 53: 1036.
- Danziger J, Wallace S, Handel S, et al. (1997). The sella tursica in primary endorgan failure. Radiology. 131: 111.
- Sarlis NJ, Brucker-Davis, Dopmann JL, et al. (1997). MRI demonstrates regression of pituitary mass in a case of primary hypothyroidism after a week of acute hormonal therapy. J Clin Endocrinol Metab. 82: 808.
- Poretsky L, Garber J, Kleefield J. (1986). Primary amenorrhea and pseudoprolactinoma in a patient with primary hypothyrpoidism. Am J Med. 81: 180.
- Kochar Kaur K, Allahbadia GN, Singh M. (2016). An update on the causes of primary and secondary amenorrhea along with aetiopathogenesis and therapeutic management. Avid Science Monograph Series. 1-43.
- Alper MM, Garner PR. (1985). Premature ovarian failure: Its relationship to autoimmune disease. Obstet Gynecol. 66: 27.
- Coulam C, Adamsen SC, Anneggers JF. (1986). Incidence of premature failure. Obstet Gynecol. 67: 604.
- Luborsky JL, Meyer P, Sowers MF, et al. (2003). Premature menopause in a multi ethnic population study of menopausal transition. Hum Reprod. 18: 199.
- Dewald GW, Spurbeck JL. (1983). Sex chromosomal anomalies associated with premature gonadal failure. Seminars Reprod Endocrinol. 1: 79.
- Devi AS, Metzger DA, Luciano AA, et al. (1998). 45X/46XX mosaicism in patients with premature ovarian failure. Fertil Steril. 70: 89.
- Prueitt RL, Chen H, Barnes RI, et al. (2002). Most X Autosome translocations associated with premature ovarian failure do not interrupt X linked genes. Cytogenet Genome Res. 97: 32.
- Schlessinger D, Herrera I, Crisponi L, et al. (2002). Genes and translocations involved in P.O.F. Am J Med Genet. 111: 328.
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. (1999). Fragile X premutaions is a significant risk factor for premature ovarian failure: the international collaborative POF in Fragile X study-preliminary data. J Med Genet. 83: 322.
- Hundsheid RD, Smits AP, Thomas CM, et al. (2003).Female carriers of fragile X permutations have no increased risk for additional diseases other than premature ovarian failure. Am J Med Genet. 117: 6.
- Schmidt T, Ovitt CE, Anlag K, et al. (2004).The murine winged – helix transcription factor FOX12 is required for the gonadal cell differentiation and ovarian maintenance. Development. 131: 933.
- Sung L, Bustillo M, Mukherjee T, et al. (1997). Sisters of women with premature ovarian failure may not be ideal ovum donors. Fertil Steril. 67: 912.
- Van Kastern YM, Braat DDM, Hemrika DJ, et al. (1998). Corticosteroids do not influence ovarian responsiveness to gonadotropins in patients with premature ovarian failure: A randomized placebo controlled trial. Fertil Steril. 71: 90.
- Aitomaki K. (1994). The genetics of XX gonadal dysgenesis. Am J Hum Genet. 54: 844.
- Aitomaki K, DieguzLucena JL, PakarinenP, et al. (1995). Mutation in the follicle stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 82: 959.
- Jiang M, Aittomaki K, Nilson C, et al. (1998). 590C –T of the human follicle stimulating hormone receptor gene in four populations using allele specific hybridization and time resolved spectroscopy. J Clin Endocrinol Metab. 83: 4338.
- Layman LC, Amde S, Cohen DP, et al. (1998). The Finnish follicle stimulating hormone receptor gene mutations in North American women with 46XX ovarian failure. Fertil Steril. 69: 300.
- Beatriz da Fontez Kohek M, Cidade Batista M, Russell AJ, et al. (1998). No evidence of the inactivating mutation (C566T) in the follicle stimulating hormone receptor gene in Brazilian women with premature ovarian failure. Fertil Steril. 70: 565.
- Takakura K, Takehayashi K, Wang HQ, et al. (2001).Follicle stimulating hormone receptor gene mutations is rare in Japanese women with premature ovarian failure and polycystic ovary syndrome. Fertil Steril. 75: 207.
- Doherty P, Pakarinen P, Titiinen A, et al. (2002). A novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J Clin Endocrinol Metab. 87: P1151.
- Tourraine P, Beau I, Gougeon A, et al. (1999).New natural inactivating mutations of the follicle receptor hormone correlations between receptor finvtion and phenotype. Mol Endocrinol. 13: 1844.
- Allen LA, Achermann JC, Pakarinin P, et al. (2003). Anovel lss of functional mutation in exon 10 of the FSH receptor gene causing hypergonadotropic hypogonadism: Clinical and molecular characteristics. Hum Reprod. 18: 251.
- Meduri G, Tourine P, Beau I, et al. (2003).Delayed puberty and primary amenorrhea associated with a novel mutation in human FSH receptor: Clinical, histological and molecular studies. J Clin Endocrinol Metab. 88: 3491.
- Toledo SPA, Brunner HG, Kraaij R, et al. (1996). An inactivating mutation of the LH receptor causes amenorrhoea in a 46XX female. J Clin Endocrinol Metab. 81: 3850.
- Delon B, Lalloiu H, Abel-Labalanche C, et al. (1997). Fluorescent in situ hybridization and sequences tagged sites for delineation of an X-Y translocationin a patient with secondary amenorrhea. Mol HumReprod. 3: 439.
- Conway GS, Payne NP, Webb J, et al. (1998).Fragile X permutation screening in women with premature ovarian failure. Hum Reprod. 13: 1184.
- Davison RM, Quilter CR, Webb J, et al. (1998). A familial case of X chromosome deletion ascertained by cytogenic screening of women with premature ovarian failure. Hum Reprod. 13: 3039-3072.
- Ebrahimi M, Asbagh FA. (2015). The role of autoimmunity in premature ovarian failure. Iran J Reprod Med. 13: 461
- Qin Y, Jiao X, Simpson L, et al. (2015). Genetics of primary ovarian insufficiency: New developments and opportunities. Hum Reprod Update. 21: 787-808.
- Chapman C, Cree L, Shelling AN. (2015).The genetics of premature ovarian failure: Current perspectives. Int J Womens Health. 7: 799-810.
- Yang X, Zhou Y, Peng S, et al. (2012).Differentially expressed plasma microRNAs in premature ovarian failure and the potential regulatory function in mir23a in granulose cell apoptosis. Soc Reprod Fertil. 144: 235-244.
- Dang Y, Zhao S, Qin Y, et al. (2015). microRNAs-22-3pis downregulated in the plasma of Han Chinese patients with premature ovarian failure. Fertil Srteril. 103: 802-807.
- Tsuiko O, Noukas M, Zilina O, et al. (2016). Copy number variation analysis detects novel candidate genes involved in follicular growth and oocyte maturation in a cohort of premature ovarian failure cases. Hum Reprod. 31: 1913-1925.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences