A review on common pathogenic microorganisms and their impact on human health
Pinky Sarmah, Meria M Dan, Dattatreya Adapa, Sarangi TK
Pinky Sarmah1, Meria M Dan2, Dattatreya Adapa3*, Sarangi TK4
1Independent Researcher, Hyderabad, India
2AMITY Institute of Biotechnology, AMITY University, UP, India
3Department of Microbiology, Food Science and Technology, GITAM University, AP, India
4Department of Biotechnology, VIT University, Tami Nadu, India
Received date: March 16, 2018; Accepted date: March 30, 2018; Published date: April 10, 2018
Citation: Sarmah P, Dan MM, Adapa D, et al. A Review on Common Pathogenic Microorganisms and Their Impact on Human Health. Electronic J Biol, 14:1
Abstract
Pathogens in hostile terms can be defined as invaders that attack host organisms. Pathogens show different types of specificity or tropism. While many can attack a wide range of hosts, others are specific to one host. A human body is a preferable host for a wide variety of pathogens as it provides nutrient rich, warm and moist environment. Moreover the uniform temperature of the human body, allows the pathogen to survive and multiply itself, making it a desirable place. Emerging Infectious diseases by various pathogens had always been a matter of concern in the past and also at present. Despite the tremendous advancement in the diagnosis, treatment and prevention procedures, these infectious diseases still remain the leading cause of death around the world especially in developing countries. In this review, we made an attempt to discuss about some of the human pathogens like Human papillomavirus, Helicobacter pylori, Hepatitis, Mycobacterium tuberculosis and Plasmodium vivax, which are listed among the lethal microorganisms that frequently infect humans.
Keywords
Human pathogen; Human papillomavirus; Helicobacter pylori; Hepatitis, Mycobacterium tuberculosis; Plasmodium vivax.
Introduction
The capacity of a microorganism to cause disease is called pathogenicity. A microorganism that has the ability to cause disease is called pathogen. There are a wide range of microorganisms that can cause severe harm to the body and become lethal [1]. In spite of the remarkable research and development in the treatment and prevention procedures, infectious disease still remains the topmost cause of death in the world, particularly in the developing countries; more virulent pathogens are continuously emerging. For example, the worldwide incidence of tuberculosis (TB) caused by the pathogen Mycobacterium tuberculosis is estimated to be ~9 million new cases (~0.1% of the global population) per annum, leading to 1.5-2 million deaths per annum and with an estimated 450,000 people getting multi-drug resistant tuberculosis per year [2]. Antimicrobial resistance is a major concern as the excessive usage of antimicrobial drugs not only making the microorganisms resistant but also causing severe infections which are harder to treat [3]. According to World Health Organization (WHO), ~1/3rd of the world's population carries a latent M. tuberculosis infection which means that there is a huge reservoir for potential future TB cases. In 22 African countries, according to WHO report (2014), more than 70% premature deaths occurred due to infectious diseases, with co-infections such as HIV and helminths. Therefore, there is a need to develop new molecular technologies and knowledge to fight against human microbial pathogens [4].
In United States, norovirus alone is responsible for ~21 million cases of acute gastroenteritis per year, which includes >70,000 hospitalizations and almost 800 deaths [5-7]. In developing countries, noroviruses causes up to 200,000 deaths every year in children below 5 years. The greatest burden of diarrheal disease occurs in these countries [8,9]. Lactobacillus plantarum (LpS2), a newly identified microorganism produces a γ-aminobutyric acid (GABA) in a large amount. GABA is a non-protein amino acid and is largely distributed in nature. It plays a key role by acting as a major inhibitory neurotransmitter in the central nervous system of mammals. GABA is responsible for causing some harmful effects such as, hypotensive, diuretic and tranquilizing effects. It is also involved in several neurological disorders, like Parkinson’s disease, Alzheimer’s disease, and Huntington’s chorea [10].
Virulence is the harm caused to the host by the pathogen. It varies from species to species ranging from negligible to immediate death. Classic theory of virulence evolution is mainly based on a trade-off between the pathogen growth-transmission and the host survival. This indicates that higher the growth within host, higher is the transmission and virulence [11]. The range of infectious diseases is evolving quickly. Evolving infectious microbes display an interesting group of nosocomial challenges [12]. Nosocomial infections are increasing worldwide, both in developed and developing countries. The ability of the pathogens to mutate frequently and emerge into more virulent form is making these pathogens resistant towards the drugs against them. For example, resistant form of Staphylococcus aureus, known as vancomycin-resistant Staphylococcus aureus (VRSA) has emerged which is resistant against the most effective antibiotic - Vancomycin. Emerging infections can be defined as infections, that are new in the population or they are already existing but rapidly increasing in incidence or in the geographical area [13]. Pathogens which are already present in the environment causes most of the emerging infections, brought out either unknowingly or by selectively taking opportunity of changing conditions and infecting new host population [14]. The process of transferring infectious agents from animals to humans or from isolated groups into new populations is known as microbial traffic [15,16].
In 2012, a strange microbe (later known to be Mycobacterium chimaera) was observed in few patients who had undergone open heart surgery. M. chimaera, within 3 years, had infected approximately 49 people around the world and half of them died. Researchers have traced the mode of transfer of infection and found it to be contaminated medical equipment’s used in heart surgery. According to a new study, this infection was originated from the device manufacturing site in Germany [17,18]. In the last four decades, the worldwide emergence and revival of arboviruses has become a rapidly growing public health crisis. Dengue virus reappeared quadrupled times more in the late 20th century from 1980s–2000s and at present, it causes approximately 96 million symptomatic cases per year [19-21]. Chikungunya virus emerged for the first time in America in St. Martin and 1.8 million suspected and confirmed cases were reported in the entire region [22]. In 2015, the first case of locally acquired Zika virus (ZIKV) was reported in Brazil [23,24]. Since then, over 500,000 suspected and confirmed cases of ZIKV has been reported in 40 countries and territories in America [25,26]. Many ZIKV cases most likely have gone unreported due to initial less reporting, high proportion of asymptomatic cases (>80%), and diagnostic challenges [27]. Similarly in 2014, Ebola virus has caused innumerable deaths in Central and West African regions especially in
Pathogen-human interaction
Specificity of a pathogen towards its host is defined as the ability of the pathogen to colonize or infect its host [29]. Various pathogens have various levels of specificity. Many bacteria have a wide range of host specificity. Such bacteria may infect insects, humans, rodents and several other wild/domestic animals. Many bacteria show a low level of specificity. These pathogens exhibit different pathogenicity between human and other hosts. For example, Salmonella typhimurium after oral ingestion causes gastroenteritis in humans, but in mice, this leads to symptoms resembling typhoid fever of human [30]. Specificity of pathogens towards its host is determined by determining the molecular interactions between the host and the pathogen. Due to relative simplicity of viral genomes and their structures, their host specificity is easy to determine. Viral specificity is predominantly defined by the interactions between the viral proteins and their cognate cellular receptors [31]. The host specificity of bacterial pathogens is less understood due to more complex molecular compositions and cellular structures. However in the last two decades, huge development has been made to completely understand the specificity of pathogenic bacteria towards its host [32].
The human body is a complex and flourishing ecosystem. It consists of 1013 human cells and also about 1014 bacterial, fungal and protozoan cells, representing thousands of microbial species [33]. These microbes are called normal flora and are usually restricted to certain parts of the body, including skin, mouth, large intestine, and vagina. In addition, humans are frequently infected with viruses, which rarely become symptomatic [33].
Normal flora is different from pathogens. Normal microbial inhabitants cause trouble only if the immune system is weak or if they get exposed to a sterile part of the body (example, when the gut flora enter the peritoneal cavity of the abdomen by bowel perforation it causes peritonitis). Whereas, pathogens can attack a normal host without being immunocompromised or injured. They can easily cross the cellular barriers, disturb the biochemical mechanisms and generate response from the host that helps the pathogen for its survival and multiplication. A successful pathogen must have the following capabilities to survive and multiply in a host: (1) colonize or invade the host (2) spot a nutritionally rich place in the host body (3) avoid, destroy or defeat the host innate and adaptive immune responses; (4) multiply, using host resources; and (5) escape and colonize new host. To complete this set of tasks, pathogens have developed specialized mechanisms to exploit the maximum resources of the host to induce the most appropriate host cell response [33].
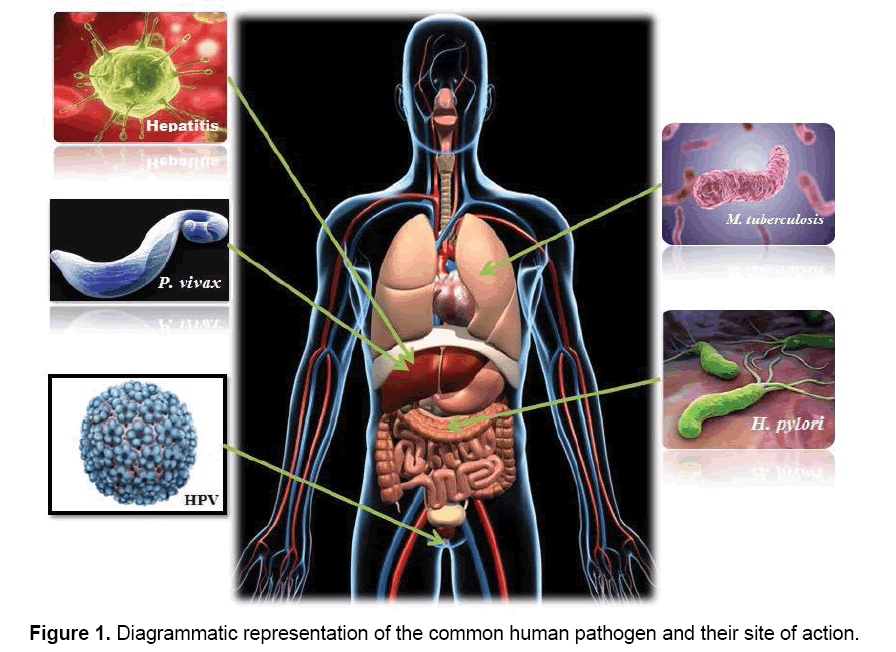
These pathogens can easily penetrate inside the targeted region within the host and rapidly replicates inside the host pathogens. They have different modes of transmission. Difference in pathogenic effects and the level of virulence depends on the host-pathogen interaction, environmental factors and host immune system. A brief summary of the mode of transmission, targeted cells and replication of the pathogens has been described in Table 1.
| Organism | Mode of Transmission | Targeted Human Cells |
|---|---|---|
| Human papillomavirus | Sexually transmitted | Basal cells of stratified epithelium |
| Helicobacter pylori | Oral-oral or fecal-oral | Mucoid lining of the stomach |
| Hepatitis | Fecal-oral route, Blood Transfusions and infected body fluids | Liver Cells |
| Mycobacterium tuberculosis | Airborne | Lung cells |
| Plasmodium vivax | Bite of an infective female anopheles mosquito | Red blood cells, Liver cells |
Table 1: Summarization of the discussed human pathogens and the targeted areas of action.
Lethal Human Pathogens
In hostile terms, pathogens or parasites are considered as invaders that attack human body, but the actual fact is they are simply trying to live and procreate like any other organism. But the only difference is they are living at the expense of a host organism in a very attractive strategy. Since human body provides the correct temperature and environment for their propagation, it is not surprising that many microorganisms have evolved the ability to survive and reproduce in this desirable niche. Different pathogens have different site of action (Figure 1), where they interfere into the host metabolic pathways for their survival and multiplication. In this section, we have taken into account few lethal pathogens which are commonly seen in humans and cause chronic diseases. The diseases caused by the above mentioned five lethal pathogens namely, Human papillomavirus, Helicobacter pylori, Hepatitis, Mycobacterium tuberculosis and Plasmodium vivax are known to be among the frequently occurring diseases and at times most infectious.
Human papillomavirus (HPV)
These viruses are tiny, non-enveloped, icosahedral with double stranded DNA. The human papillomavirus (HPV) is a DNA tumor virus and species specific. It infects host basal epithelial cells of skin and mucous membranes and cause different types of warts and anogenital cancers [34]. More than 100 different types of the HPV exist, and ~30 to 40 strains infect genital tract of humans. Out of these, the oncogenic or high-risk types (16, 18, 31, 33, 35, 39, 45, 51, 52 and 58) causes cervical, vulvar, vaginal and anal cancers and non-oncogenic or low-risk types (6, 11, 40, 42, 43, 44 and 54) causes genital warts [35]. HPV 16 is the most cancerous type, responsible for almost half of all cervical cancers, and HPV 16 and 18 together are responsible for 70% of cervical cancers [36]. HPV 6 and 11 are commonly associated with genital warts and account for approximately 90% of these lesions [37]. Globally cervical cancer is a major cause of illness and death among women, and HPV being the principal cause [38,39]. Presumably, the major reservoir for genital HPV infection for women is men [40].
Following endorsement from national and international advisory bodies, in most of the developing and developed countries, HPV vaccines have been introduced widely. HPV vaccine is the first developed vaccine against cancer and it is gender specific, which makes it unique and challenging [41]. It is made to target those adolescent females who are not reached by any health intervention programs [42]. Gardasil®, Gardasil® 9 and Cervarix® are the three vaccines approved by Food and Drug Administration (FDA) to prevent HPV infection. These vaccines are active against new HPV infections, but they cannot provide protection against established HPV infections or disease caused by HPV [43,44]. Currently there is no medical treatment for persistent HPV infections which are not associated with abnormal cell changes [45]. However, there is treatment for genital warts, benign respiratory tract tumors, precancerous changes at the cervix and cancers resulting from HPV infections [46].
Helicobacter pylori
Helicobacter pylori (H. pylori) is a helix-shaped gram negative bacterium, about 3 μm in length with a diameter of about 0.5 μm. It is microaerophilic which means it requires oxygen [47] and it causes chronic infection [48]. In most cases, it is acquired in childhood, and is mostly associated with low income class [49,50]. The presence of this bacterium is the major cause for several gastroduodenal diseases, including peptic ulcer disease [51], gastric carcinoma and gastric MALT lymphoma [52,53].
H. pylori infection is known to be contagious. Even though, the actual route of transmission is not clear but it is assumed to be transmitted from person to person either by oral-oral or fecal-oral route, as the bacteria have been isolated from feces, saliva and dental plaque of some infected people [54]. Transmission could be observed mainly within families in developed countries and in communities in developing countries. Drinking waste-tainted water contaminated with fecal matter may also transmit H. pylori orally. Therefore a hygienic environment must be maintained to decrease the risk of H. pylori infection [55].
With the improved living standards, incidence of H. pylori infection is decreasing in many countries [56]. The prevalence of this bacterium is still appearing, especially in the Far East [57]. Intrafamilial transmission is the main mechanism of spread in most regions [58]. Socioeconomic status and hygiene level is the major determinants of the prevalence of H. pylori infection in developing countries. Until now, the global and regional prevalence of H. pylori has not been reported systematically. H. pylori infection still continues to be a major public health problem throughout the world. According to the global systematic review, in 2015, about 4.4 billion individuals globally were found to be positive for H. pylori. This is the most comprehensive and up to date systematic report of the global prevalence of H. pylori. Hooi et al. [59] studied and observed a wide difference in the prevalence of H. pylori between different regions and countries. It is highest in Africa (79.1%), Latin America, Caribbean (63.4%) and Asia (54.7%). On the other hand, H. pylori prevalence is lowest in Northern America (37.1%) and Oceania (24.4%).
Inspite of H. pylori being sensitive to a wide variety of antibiotics in vitro, they all are ineffective as monotherapy in vivo. Clarithromycin is the most effective drug which can eradicate 40% of infection, when given twice daily for 10 to 14 days [60-62]. Due to the niche of H. pylori which can survive at lower pH and resides in a viscous mucus layer, there is a lack in the efficacy of monotherapy. Different types of therapies are in use to treat this infection, such as dual, triple, and quadruple therapy. In dual therapy, a combination of twice-daily-dosed proton pump inhibitor (PPI) and amoxicillin (antibiotic) is used. Dual therapies have been replaced by triple therapies in almost everywhere. This therapy uses the combination of two antibiotics with either a bismuth compound or a proton pump inhibitor (PPI). Quadruple therapy is a further alternative, where the bismuth compound and PPI are used in combination with two antibiotics [63].
Hepatitis
Hepatitis virus mainly affects liver and causes inflammation. There are five major unrelated types of hepatitis virus exists: hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV) and hepatitis E virus (HEV). HAV, HCV, HDV and HEV are RNA viruses, whereas HBV is a double stranded DNA virus. The route of transmission of HAV and HEV is the fecal-oral route and mostly observed in areas with poor sanitation and impure drinking water. The host immune system can clear the acute infection caused by these viruses. HBV and HCV are the major viruses of chronic hepatitis and liver disease that can lead to hepatocellular carcinoma. HBV resides in blood, semen and other body fluids and it is transmitted through intercourse, reused injections or perinatally. HCV is mainly transmitted through blood [64,65].
According to most recent data from Centre for Disease Control and Prevention (CDC), HAV accounts for approximately 1,781 new infections each year. HAV causes acute hepatitis and it never becomes chronic. Hepatitis A is contagious and earlier referred to as “infectious hepatitis” as it easily gets transmitted from person to person. Hepatitis A virus can be transmitted through the ingestion of contaminated food or water, especially where unhygienic sanitary conditions make water or food contaminated with human waste containing hepatitis A (the fecal-oral mode of transmission) [66].
More than 19,000 new cases of HBV infection has been estimated by CDC in 2013 and in United States, more than 1,800 people die per year due to chronic hepatitis B infection. HBV hepatitis was earlier called as "serum hepatitis," because it was assumed that HBV could spread only through blood or serum carrying the virus. It is now known that HBV can be transmitted by sexual contact, transfer of infected blood or serum through shared needles among drug addicts, accidental needle pricks contaminated with infected blood, blood transfusion, hemodialysis and by infected mothers to their babies. According to CDC report, there were approximately 16,500 new cases of hepatitis C reported each year (unreported is 13.4 times more) [66].
HCV is a global burden and approximately 90% of transfusion-associated hepatitis is caused by HCV [67,68]. Transmission through sexual contact is rare. In U.S. there are approximately 3.2 million people with chronic HCV infection [66]. Although associated with toxicities and low sustained viral response rates, the mainstay of treatment is interferon alfa and ribavirin until date. New direct acting antivirals, specifically designed to inhibit three viral proteins (the NS3/4A protease, the NS5A protein, and the NS5B RNA dependent RNA polymerase) are now becoming available. As per the reports from WHO, nearly 350,000-500,000 people die from HCV-related complications every year [69].
About 15 million people globally are infected with hepatitis D virus. HDV requires HBV infection for its replication. It is known as “defective satellite virus” as it can multiply only in HBV infected cells. Patients co-infected with Hepatitis B and D is more prone to have serious affects than those infected with Hepatitis B alone. Hepatitis E virus (HEV) is very similar to Hepatitis A in terms of clinical course and mode of transmission. Many large outbreaks have been observed in refugee camps. The mortality rate of HEV infection is 1% to 2%, about 10 times more than that of HAV. HEV infection is more vulnerable for pregnant women (mortality of ~20 %). HGV also called as GB-C virus is a recently defined virus that is after the initials of the surgeon who have first isolated this virus. It is still not clear whether it is a reason for maximum liver disease. It is mostly found in patients infected with other chronic hepatitis viruses which make it difficult to determine the effect [70].
According to CDC, routine childhood vaccination of hepatitis A implemented in many parts of United States in 1990s has decreased new cases of Hepatitis A by 95 percent between 1995 (12 cases per 100,000 people) to 2010 (less than 1 case per 100,000 people). According to WHO, Hepatitis B vaccination can effectively prevent 95 percent viral infections and its chronic consequences. Hepatitis B vaccination can also prevent hepatitis D. There is no vaccination for hepatitis D. As per NIH, hepatitis B infected mothers giving birth to newborn should be given hepatitis B immunoglobulin and the hepatitis B vaccine within 12 h of birth to prevent infection [71].
Mycobacterium tuberculosis
Mycobacterium tuberculosis is a member of M. tuberculosis complex (MTBC) that consists of six other closely related species: M. bovis, M. africanum, M. microti, M. pinnipedii, M. caprae and M. canetti.
The genome of M. tuberculosis is <0.05% different from M. bovis. According to a recent molecular biology study, the most common cause of tuberculosis, i.e., M. tuberculosis has ~3 million years old progenitor [72].
According to WHO report (2007), 9.27 million new active disease cases corresponding to an estimated incidence of 139 per 100,000 populations occurred throughout the world [73]. The highest number of TB cases were reported in Asia (55%) followed by Africa (31%). The highest incidence (363 per 100,000 populations) was recorded in Africa, mainly because of the high prevalence of HIV infection [74]. The six most populous countries of Asia (China, India, Indonesia, Pakistan, Bangladesh and Philippines) accounted for more than 50% of TB cases globally [75].
Tuberculosis is mainly transmitted by inhalation of aerosol droplets exhaled by infected hosts and it is highly infectious. There are about 1-400 bacilli per aerosol droplet and the infectious dose is 1-200 bacilli which makes contact without infection almost impossible. M. tuberculosis uses the host defence system for its own benefit. The patient will either get a primary infection immediately after the attack or the disease remains silent within the host body. When inhaled, tuberculosis bacteria enter the lungs and finally enter the alveoli. In the alveoli, these are considered as foreign molecules by the immunocompetent host and are attacked by the macrophages [76].
Some strains of M. tuberculosis are more virulent than others as they show increased transmissibility and more mortality and morbidity rate in infected patients [77]. In spite of the broad use of an attenuated live vaccine and different antibiotics, the occurrence of TB is increasing and thereby requires the development of new vaccine and medicines. Diagnosis of TB also needs more specific and rapid technologies [78]. Despite the widespread use of an attenuated live vaccine and several antibiotics, there is more TB than ever before, requiring new vaccines and drugs and more specific and rapid diagnostics. It is among the oldest human afflictions and continues to be one of the biggest killers among all the infectious diseases [79].
Bacillus Calmette-Guerin (BCG) was the first vaccine against TB, developed in the 1920s. It is used by almost 80% of all the newly born babies and children as a part of the national childhood immunization program 1. It is one of the most widely used vaccines. BCG vaccine provides excellent protection to children against disseminated forms of TB, but protection to adults against pulmonary TB is variable. As most transmission mostly originates from adult cases of pulmonary TB, BCG vaccine is normally used to protect children, rather than to interrupt transmission among adults [80]. Even after the initiation of the treatment, pulmonary tuberculosis is contagious till two to three weeks. Patients were isolated earlier but these days as isolation is not practiced, the following are the few precautions to prevent transmission:
• Isolation from work areas, schools, colleges and crowded areas.
• The infected person must cover his or her mouth and nose while coughing or sneezing.
• Careful and proper disposal of used tissues. Burning or disposal in sealed plastic bags is highly recommended.
• Sharing of beds or rooms with normal or uninfected persons while sleeping must be avoided [81].
As we had already discussed that BCG being the first and the foremost effective drug against TB has a variable effect on adults. Since the treatment regime in patients is a time consuming process and often results in the occurrence of drug resistant TB (Multi-drug and extremely drug resistant TB). This has been a matter of concern in several regions of the world. As per TB vaccine R&D more than 10-15 drug candidates are still at various levels of trials for vaccine development focusing more on adult vaccine than a child vaccine [82]. It was also mentioned that out of 15 candidates in the active clinical development containing the same handful antigen combination, only 12 were targeted among the 4,500 antigens encoded in the MTB genome along with viral-vectored and protein/adjuvant. This targets the greatest need for the development and improvement of these drugs [83].
Plasmodium vivax
Vivax malaria was earlier called as ‘benign tertian malaria’ as single clinical episodes were less likely to cause serious illness compared to Plasmodium falciparum. Considered as a benign infection for a long time, P. vivax has been now recognized as the cause of severe and lethal malaria. Although the parasite biomass is low, increasing deformation of the infected red blood cells and an apparent paucity of parasite sequestration are making it lethal. Inspite of this, Plasmodium vivax (P. vivax) was and still remains the major cause of morbidity and mortality in vivax endemic regions. Repeated or chronic infections in vivax endemic areas can cause severe malnutrition and anaemia, specifically in early childhood. Other severe diseases or manifestations are acute lung injury, acute kidney injury and uncommonly, coma [84].
Severe anemia is related with repeating incidents of hemolysis of predominantly uninfected red blood cells and increasing fragility. Lung injury is due to increased inflammation in alveolar-capillary membrane permeability [85]. Severe vivax anaemia causes substantial indirect mortality and morbidity by irreversible resilience to co-morbidities such as obstetric complications and demand for blood transfusion. Vivax or falciparum infected patients subsequently develops bone marrow abnormalities because of impaired erythropoiesis. In the initial stages of both the types of infections, the typical marrow abnormalities are because of decreased cellularity [86]. In those with severe chronic infections, marrow cellularity tends to be normal or increased but there erythropoiesis does not occur normally [87], indicating impaired iron utilization [88], presence of morphologically impaired erythroblasts due to cellular injury [89] and phagocytosis of erythroblasts by bone marrow macrophages [90].
Plasmodium vivax infects 19-50 million people with malaria each year. Most of these cases occur in the Asia-Pacific region where more than 2.2 billion people are at risk of infection. Since 1960, only five countries in the Asia-Pacific have been certified free from malaria: Taiwan (1965), Australia (1981), Singapore (1982), Maldives (1984), Brunei (1987). Several factors contribute to this impact. P. vivax can be difficult to detect because it usually circulates at low levels in the blood. It could be infectious in the form of mosquito vectors, even when the person shows no symptoms [91]. P. vivax can also lie dormant in a person’s liver, reawakening weeks to months after the first infection to cause relapses of symptomatic malaria [92].
The aims of antimalarial treatment against P. vivax are to decrease the immediate risk for the host, remove peripheral asexual parasitemia, inhibit the repeating infection and prevent the cycle of transmission [93,94]. The capability of P. vivax to form dormant liver stages (hypnozoites), is the main cause of relapsing of infections weeks to months after the initial bloodstage infection, makes it a major challenge for the complete eradication of parasites from the host body. As monotherapy using single drug could not achieve all of these aims, a combination of antimalarials is important which can target a variety of targeted key elements of the parasite life cycle [95,96]. The basic controlling measures for P. vivax malaria are similar to those for P. falciparum malaria:
• Prevent the transmission of the parasite by taking measures to control and kill the vector mosquitoes.
• Prevent the progress of infection in human beings by chemoprevention.
• Rapid detection, diagnosis and treatment by providing accessible and efficient diagnostic testing and treatment services [97,98].
Conclusion
Human pathogens are socioeconomic burden and serious concern for public health. Even though the existing drugs and vaccines are helping in preventing these infectious diseases up to an extent but still there is a need to develop advanced treatments [99]. In the present scenario, the treatment regimens against various infectious diseases are causing microbial resistance toward the drug. The increasing drug resistance is partly due to the frequent mutation of the pathogens and partly because of the overuse or misuse of drugs [100,101]. There are many ways by which a pathogen escapes the host defence mechanism and causes infection. Antigenic variation and latency are the most common way of evading. Some pathogens can use the host immune response for spreading the infection. Thus, requires the urgency in developing new diagnostic approaches to identify the infection at the initial stages in order to subsidize the proliferation of the disease to a chronic infection. As these microbes are continuously mutating, more virulent forms are coming into the environment. Maintaining a hygienic atmosphere in the developing countries to reduce the prevalence of these harmful microbes can further help to control the emergence of these infectious diseases. Infectious diseases have always had the upper hand and continue to surprise us. They are often unpredictable and can leave us with no treatment or vaccine on hand. Continual research in the public health sphere is also an essential need of the time.
Acknowledgement
We would like to acknowledge and express our gratitude to Dr. Amit Banerjee for his support and invaluable suggestions.
References
- https://microbiologyonline.org/about-microbiology/microbes-and-the-human-body/microbes-and-disease
- Sarmah P, Dan MM, Adapa D. (2017). Antimicrobial resistance: A tale of the past becomes a terror for the present. Electronic J Biol. 13: 420-26.
- Ali Abdullah AM, AhmedGhalib AA. (2017). Pattern of antimicrobial prescribing among in-patients of a teaching hospital in Yemen: A prospective study. Universal Journal of Pharmaceutical Research. 2: 11-17.
- Soares NC, Bou G, Blackburn JM. (2016). Proteomics of microbial human pathogens. Front Microbiol. 7.
- Scallan E, Hoekstra RM, Angulo FJ, et al. (2011). Foodborne illness acquired in the United States—Major pathogens. Emerg Infect Dis. 17: 7.
- Lopman BA, Hall AJ, Curns AT, Parashar UD. (2011). Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996-2007. Clin Infect Dis. 52: 466-474.
- Hall AJ, Curns AT, McDonald LC, et al. (2012). The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999-2007. Clin Infect Dis. 55: 216-223.
- Patel MM, Widdowson MA, Glass RI, et al. (2008). Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 14: 1224-1231.
- Hall AJ. Noroviruses: The perfect human pathogens?
- Sui C, Liang J, Liu J. (2016). Cloning and bioinformatics analysis of a glutamate decarboxylase from Lactobacillus plantarum LpS2. Biomed Res. 27: 298-304.
- Leggett HC, Cornwallis CK, Buckling A, et al. (2017). Growth rate, transmission mode and virulence in human pathogens. Phil Trans R Soc B. 372: 20160094.
- Dattatreya A, Dan MM, Sarangi T, et al. (2017). Translational approach in emerging infectious disease treatment: An update. Biomed Res. 28: 5678-5686.
- Morse SS, Schluederberg A. (1990). Emerging viruses: the evolution of viruses and viral diseases. J Infect Dis. 162: 1-7.
- Morse SS. (1991). Emerging viruses: defining the rules for viral traffic. Perspect Biol Med. 34: 387-409.
- Morse SS. (1990). Regulating viral traffic. Issues Sci Technol. 7: 81-4.
- Morse SS. (2001). Factors in the emergence of infectious diseases. In: Plagues and Politics, Palgrave Macmillan UK. 8-26.
- https://www.sciencemag.org/news/2017/07/we-may-now-know-how-rare-lethal-microbe-infected-dozens-people-across-globe
- Arora N, K Banerjee A. (2012). New targets, new hope: Novel drug targets for curbing malaria. Mini Rev Med Chem. 12: 210-26.
- Gubler DJ. (2002). Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 10: 100-103.
- Gubler DJ. (1998). Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 11: 480-496.
- Bhatt S, Gething PW, Brady OJ, et al. (2013). The global distribution and burden of dengue. Nature. 496: 504-507.
- PAHO WHO. (2016). Chikungunya. Statistic data [Internet]. https://www.paho.org/hq/index.php?option=com_topics&view=readall&cid=5927&Itemid=40931&lang=en
- Kindhauser MK, Allen T, Frank V, et al. (2016). Zika: The origin and spread of a mosquito-borne virus. Bull World Health Organ. 94: 675-86C.
- Arora N, Amit KB, Mangamoori LN. (2016). Zika virus: An emerging arboviral disease. 395-399.
- Pan American Health Organization/World Health Organization. (2016). Zika epidemiological update. Washington, D.C.: PAHO/WHO.
- Pan American Health Organization/World Health Organization. (2016). Cumulative Zika suspected and confirmed cases reported by countries and territories in the Americas, 2015-2016. [Internet]. Washington, D.C.: PAHO/WHO.
- Ali S, Gugliemini O, Harber S, et al. (2017). Environmental and social change drives the explosive emergence of Zika virus in the Americas. PLOS Negl Trop Dis. 11: e0005135.
- Raj KK, Anil KS, Urmila J, et al. (2016). Ebola virus disease and its complications. Universal Journal of Pharmaceutical Research. 1: 54-58.
- Kirzinger MW, Stavrinides J. (2012). Host specificity determinants as a genetic continuum. Trends Microbiol. 20: 88-93.
- Bäumler A, Fang FC. (2013). Host specificity of bacterial pathogens. Cold Spring Harb Perspect Med. 3: a010041.
- Medina RA, García-Sastre A. (2011). Influenza A viruses: New research developments. Nat Rev Microbiol. 9: 590-603.
- Pan X, Yang Y, Zhang JR. (2014). Molecular basis of host specificity in human pathogenic bacteria. Emerg Microbes Infect. 3: e23.
- Alberts B, Johnson A, Lewis J, et al. (2002). Molecular Biology of the Cell. Garland Science, 4th edn, New York.
- Sabeena S, Bhat P, Kamath V, et al. (2017). Possible nonÃÆâÃâââ¬ÃâÃÂsexual modes of transmission of human papilloma virus. J Obstet Gynaecol Res. 43: 429-435.
- Muñoz N, Bosch FX, de Sanjosé S, et al. (2003). Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003: 518-27.
- Clifford GM, Smith JS, Aguado T, et al. (2003). Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: A meta-analysis. Br J Cancer. 89:101-105.
- Braaten KP, Laufer MR. (2008). Human papillomavirus (HPV), HPV-related disease and the HPV vaccine. Rev Obstet Gynecol. 1: 2.
- Schiffman MH. (1992). Recent progress in defining the epidemiology of human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst. 84: 394-398.
- Bosch FX, De Sanjosé S. (2003). Human papillomavirus and cervical cancer — Burden and assessment of causality. J Natl Cancer Inst. 2003: 3-13.
- Hernandez BY, Wilkens LR, Zhu X, et al. (2008). Transmission of human papillomavirus in heterosexual couples. Emerg Infect Diseases. 14: 888.
- Muhammad H. (2015). The knowledge about, beliefs and attitudes of medical students regarding vaccination against the human papillomavirus, in South Africa: A cross-sectional study. Biomed Res. 26: 65-70.
- Basu P, Banerjee D, Singh P, et al. (2013). Efficacy and safety of human papillomavirus vaccine for primary prevention of cervical cancer: A review of evidence from phase III trials and national programs. South Asian J Cancer. 2: 187.
- Hildesheim A, Herrero R, Wacholder S, et al. (2007). Effect of human papillomavirus 16/18 L1 virus like particle vaccine among young women with pre-existing infection: A randomized trial. JAMA. 298: 743-753.
- Schiller JT, Castellsagué X, Garland SM. (2012). A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 30: 123-138.
- Güçkan R, Kilinç Ç, Gözdemir E, et al. (2016). Prevalence and distribution of high-risk human papillomavirus in Amasya region, Turkey. Biomed Res. 27: 769-772.
- https://www.paho.org/hq/index.php?option=com_topics&view=readall&cid=5927&Itemid=40931&lang=en
- Mehmood A, Akram M, Ahmed A, et al. (2010). Helicobacter pylori: An introduction. IJACPT. 1.
- Leone N, Pellicano R, Brunello F, et al. (2003). Helicobacter pylori seroprevalence in patients with cirrhosis of the liver and hepatocellular carcinoma. Cancer Detect Prev. 27: 494-497.
- Mendall MA, Goggin PM, Molineaux N, et al. (1992). Childhood living conditions and Helicobacter pylori seropositivity in adult life. Arch Dis Child. 339: 896-897.
- Queiroz DM, Carneiro JG, BragaÃÆâÃâââ¬ÃâÃÂNeto MB, et al. (2012). Natural history of Helicobacter pylori infection in childhood: Eight year followÃÆâÃâââ¬ÃâÃÂup cohort study in an urban community in northeast of Brazil. Helicobacter. 17: 23-29.
- Hopkins RJ, Girardi LS, Turney EA. (1996). Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: A review. Gastroenterology. 110: 1244-1252.
- Wotherspoon AC, Diss TC, Pan L, et al. (1993). Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 342: 575-577.
- Mosa TE, El-Baz HA, Mahmoud MS, et al. (2013). Helicobacter pylori sero-prevalence in different liver diseases. Int J Med Sci. 5: 414-419.
- Rastogi M, Rastogi D, Singh S, et al. (2015). Prevalence of Helicobacter pylori in asymptomatic adult patients in a tertiary care hospital: A cross sectional study. Biomed Res. 26: 117-122.
- Nagy P, Johansson S, Molloy-Bland M. (2016). Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 8: 8.
- Graham DY. (2014). History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 20: 5191.
- Yokota SI, Konno M, Fujiwara SI, et al. (2015). Intrafamilial, preferentially motherÃÆâÃâââ¬ÃâÃÂtoÃÆâÃâââ¬ÃâÃÂchild and intraspousal, Helicobacter pylori infection in japan determined by mutilocus sequence typing and random amplified polymorphic DNA fingerprinting. Helicobacter. 20: 334-342.
- Hooi JK, Lai WY, Ng WK, et al. (2017). Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology.
- Graham DY, Opekun AR, Klein PD. (1993). Clarithromycin for the eradication of Helicobacter pylori. J Clin Gastroenterol. 16: 292-294.
- Megraud F. (1995). Rationale for the choice of antibiotics for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 7: S49-S54.
- Peterson WL, Graham DY, Marshall B, et al. (1993). Clarithromycin as monotherapy for eradication of Helicobacter pylori: A randomized double-blind trial. Am J Gastroenterol. 1: 88.
- Kusters JG, van Vliet AH, Kuipers EJ. (2006). Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 19: 449-490.
- Louten J. (2016). Hepatitis viruses. Essential Human Virology, Chapter 12.
- Adapa D, Sai YR, Anand SY, et al. (2011). A brief review on immune mediated diseases. J Clin Cell Immunol. 11: S1-7.
- https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-fact-sheet
- Abbas MA, Arwa MO, A-Kasem MA, et al. (2017). Sero-prevalence of hepatitis C virus among dental clinic workers in Sana'a city-Yemen and the risk factors contributing for its infection. Universal Journal of Pharmaceutical Research. 2: 28-33.
- Rkem Yaman G, Erko R. (2015). Distribution of hepatitis C virus genotypes in patients with chronic hepatitis C infection in eastern Turkey. Biomed Res. 26: 697-701.
- Feeney ER, Chung RT. (2014). Antiviral treatment of hepatitis C. BMJ. 7: 349:g3308.
- https://www.medicinenet.com/viral_hepatitis/article.htm
- https://infectionnet.org/notes/hepatitis-viruses/
- Gutierrez MC, Brisse S, Brosch R, et al. (2005). Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog. 19: e5.
- World Health Organization. (2009). Global tuberculosis control: Epidemiology, planning, financing: WHO report.
- Zhu C, Liu S, Zhai J, et al. (2016). Clinical and pathological features of three types of peritoneal tuberculosis: A single centre in China. Biomed Res. 27: 1302-1308.
- Ahmad S. (2010). Pathogenesis, immunology and diagnosis of latent Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011: 1-17.
- https://www.livescience.com/34735-hepatitis-symptoms-treatment.html
- Singh SD, Masood T, Sabharwal RK, et al. (2015). Biochemical and molecular characterization of cerebrospinal fluid for the early and accurate diagnosis of Mycobacterium tuberculosis. Biomed Res. 26: 426-430.
- Bîrlutiu V, Birlutiu RM, Zaharie IS. (2016). Kikuchi disease associated with Group C Streptococcus: A case report. Biomed Res. 27.
- Smith I. (2003). Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev. 16: 463-496.
- https://www.intellectualventureslab.com/invent/overview-of-tuberculosis-pathogenesis
- https://www.tbfacts.org/tb-prevention/
- Beg S, Gaur P, Mishra S. (2017) New drugs and vaccines for tuberculosis. Recent patents on anti-infective drug discovery.
- Frick M. (2015). The tuberculosis vaccines pipeline: A new path to the same destination? 2015 Pipeline Report. 163.
- Anstey NM, Douglas NM, Poespoprodjo JR, et al. Plasmodium vivax: Clinical spectrum, risk factors and pathogenesis. Adv Parasitol. 80: 151-201.
- Anstey NM, Russell B, Yeo TW, et al. (2009). The pathophysiology of vivax malaria. Trends Parasitol. 25: 220-227.
- Weatherall DJ, Abdalla S, Pippard MJ. (2009). The anaemia of Plasmodium falciparum malaria. In: Malaria and the red cell. Ciba Fondation Symposium. 94: 74-97.
- Abdalla S, Weatherall DJ, Wickramasinghe SN, et al. (1980). The anaemia of P. falciparummalaria. Br J Haematol. 46: 171-183.
- de Mast Q, Syafruddin D, Keijmel S, et al. (2010). Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematol. 95: 1068-1074.
- Wickramasinghe SN, Looareesuwan S, Nagachinta B, et al. (1989). Dyserythropoiesis and ineffective erythropoiesis in Plasmodium vivax malaria. Br J Haematol. 72: 91-99.
- Douglas NM, Anstey NM, Buffet PA, et al. (2012). The anaemia of Plasmodium vivaxmalaria. Malar J. 11: 135.
- Dkhil MA, Delic D, Al-Quraishy S. (2016). Intestinal oxidative damage and mucin regulated gene response to Plasmodium chabaudi malaria infection. Biomed Res.27: 60-64.
- https://www.news-medical.net/health/Tuberculosis-Prevention.aspx
- Baird KJ, Maguire JD, Price RN. (2012). Diagnosis and treatment of Plasmodium vivax malaria. Adv Parasitol. 80: 203-270.
- Banerjee AK, Arora N, Murty US. Aspartate carbamoyl transferase of Plasmodium falciparum as a potential drug target for designing anti-malarial chemotherapeutic agents. Med Chem Res. 21: 2480-93.
- Baird JK, Valecha N, Duparc S, et al. (2016). Diagnosis and treatment of Plasmodium vivax malaria. Am J Trop Med Hyg. 95: 35-51.
- Banerjee AK, Arora N, Murty US. (2009). Structural model of the Plasmodium falciparum thioredoxin reductase: A novel target for antimalarial drugs. J Vector Borne Dis. 46: 171.
- WHO. (2015). Control and elimination of Plasmodium vivax malaria: Technical brief.
- Ravishankar MS, Mohan ME, Ramesh TP. (2015). Spectral presentation of Plasmodium falciparum malaria in rural Karnataka (Southern India). Biomed Res. 26: 561-566.
- Arora N, Kumar Banerjee A. (2012). Editorial [Hot Topic: Looking Beyond the Obvious: Search for Novel Targets and Drugs for Reducing the Burden of Infectious Diseases (Guest Editor: Neelima Arora)]. Mini Rev Med Chem. 12: 185-86.
- Dan MM, Sarmah P, Vana DR, et al. (2018). Wound healing: Concepts and updates in herbal medicine. Int J Med Res Health Sci. 7: 170-181.
- Mulligan ME, Murray-Leisure KA, Ribner BS, et al. (2007). Methicillin resistant Staphylococcus aureus. Am J Med. 94: 313-328.
- Piddock LJV, Wise R. (1989). Mechanisms of resistance to quinolones and clinical perspectives. J Antimicrob Chemother. 23: 475-480.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences