A Novel Enzymatic Method for Production of L-theanine
Jiayou Li, Liyun Guo, Shaosong Qian, Zhaolan Li, Qingcai Jiao
State Key Laboratory of Pharmaceutical Biotechnology,Nanjing University,Nanjing 210093,China
Abstract
L-theanine (γ-glutamylethylamide) is the main free amino acid component of tea and its favorable physiological effects on mammals have been reported. An enzymatic method for optically pure L-theanine production with a new L-aminoacylases-production fungi Cunnighamella echinulata 9980 was developed. The optimum conditions of reaction were: 0.05 M N-Acyl-DL-theanine, 0.04 g/ml pellets, 5×10-4CoCl2, pH7. This was the first report that Cunnighamella echinulata was the source of aminoacylases, and aminoacylase, regardless of its source, was applied in DL-theanine resolution.
Keywords
L-theanine; N-Acyl-DL-Theanine; Cunnighamella echinulata 9980; L-aminoacylase; optical resolution
1. Introduction
Theanine (γ-glutamylethylamide),which was first isolated and identified by Sakato [1] in the late 1940s,is the main free amino acid component of tea (more than 50%). The content of theanine in tea is between 1.5% and 3% (on average) of the dry weight [2,3]. The other natural source of theanine so far discovered was the mushroom Xerocomus badius [4]. In general,a higher grade of green tea contains more theanine [3,5]. Regarding the physiological functions of theanine,many researchers reported significant reduction in blood pressure in spontaneously hypertensive rats after some amounts of tea extract were ingested [6],inhibit the convulsive action [7] and spontaneous activity [8] caused by caffeine administration。It had also been shown that the oral intake of theanine causes a feeling of relaxation in human volunteers [9]. Its enhancing effects on the antitumor activity of adriamycin (doxorubicin) had also been reported [10-12].
Because of its good taste and favorable physiological effects on mammals,theanine could be a new food additive and several investigators had studied its effective production. Its chemical synthetic method had been reported,but it requires the protection and deblocking of reactive groups [1]. The production of theanine from Glu and ethylamine through biocatalysis was abandoned because of the low reactivity and the difficulty in pH control of the reaction mixture [13]. It took 4 weeks to produce theanine by fermentation of Camellia sinensis [14,15]. Enzymatic production of theanine reported previous was low productivity [16],many developments were made by Abelian,et al.[17,18] A industial scal preparation of L-theanine was first established in Sun Co.[19].
A novel enzymatic method for the optical resolution of DL-theanine with L-aminoacylaseproducing fungi Cunnighamella echinulata was reported. Aminoacylase (AC,N-acyl-L-amino acid amidohydrolase,E.C. 3.5.1.14),a readily available and inexpensive enzyme,are mainly used in the industrial production of enantiopure L-amino acids from their N-acetyl derivatives. Of several strains exhibiting aminoacylase activity which we isolated from local samples,a strain designated 9980 was selected for further investigation,since it was found to produce a large amount of L-theanine from the substrate of N-Acyl-DL-theanine. It was also the first time that aminoacylase,regardless of its source,was applied in DL-theanine resolution.
The factors that affect the efficiency of biocatalysis were variable and largely unexplained,and one aim of this study was to find the conditions for optimal enzyme activity and develop an efficient biocatalytic system.
2. Materials and methods
2.1 Chemicals
DL-theanine and N-acyl-DL-theanine were synthesized in our laboratory,and other reagents were analytical grade.
2.2 Acylation of DL-theanine
N-acetyl-DL-theanine was prepared by acylation of DL-theanine with acetic anhydride in an alkaline aqueous solution. Thus,16.5ml of acetic anhydride was added dropwise to the aqueous solution,containing 0.1mol of DL-theanine at pH 8-9 under 4°C. The reaction mixture was stirred for about 1.5h and then the pH value adjusted to 2-3. The solution was evaporated to dryness under vacuum,desalted with absolute ethyl alcohol,then evaporated alcohol again and added acetone. The precipitated product was filtered,and dried. The yields were in the range of 80-85% (w/w).
2.3 Microorganism
Cunnighamella echinulata 9980 used as the microbial source of aminoacylase was grown at 28°C aerobically in a 250 ml Erlenmeyer flask containing 100 ml of standard medium shaking at 135 rpm. One liter standard medium contained 20 g of glucose,10 g of polypeptone,1 g of yeast extract,2 g of N-acetyl-DL-theanine,2 g of K2HPO4,0.5 g of MgSO4,pH 6.5-7.0. After 36h-incubation,the pellets of mycelium were harvested in a centrifuge at 4000 rpm for 20 min,and then washed twice with distilled water and preserved for use.
2.4 Activity of aminoacylase
For the bioconversion assay,the reaction mixture,containing some concentration of Cunnighamella echinulata 9980 hydrated biomass and 5 mM Nacyl- DL-theanine,pH 7.0,was incubated at 37°C,200 rpm,normally for 30h in the test of aminoacylase activity. The reaction mixtures were then centrifuged and the supernatant was analysed for L-theanine. The aminoacylase activity of a defined amount of resting cells was measured as the initial L-theanine production rate in μmol g-1 wet cell h-1.
For investigation on the effect of various factors on aminoacylase activity,reaction systems were incubated under biocatalytic assay conditions as described above,but with varying levels of the detected factor. The optical level of factor was showed in the data distinctly.
2.5 Measurement of L-theanine
The concentration of L-theanine was measured with spectrophotometry. A mixture of 1ml theanine solution (with different concentration from 0 to 0.3mmol),1ml of acetate buffer (pH 5.4) and 1ml of ethanolic ninhydrin solutions was incubated at 100°C for 15 min. After reaction,the absorption spectrum of 570 nm was obtained to find out the relationship of Abs and concentration. The concentration of the reaction mixture were calculated from the calibration curve obtained by this method.
3. Results and Conclusion
3.1 Effects of medium and culture time on the biocatalysis
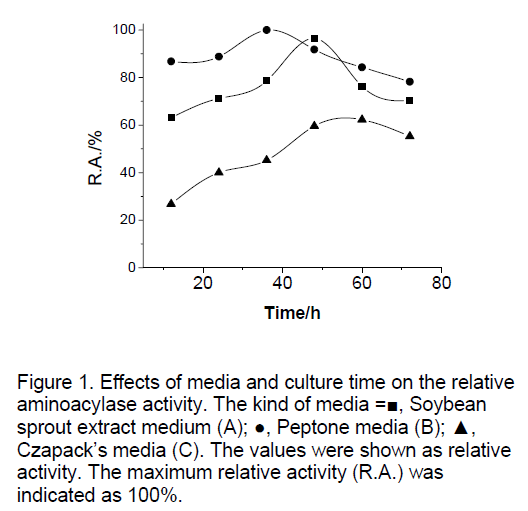
Effects of the kind of medium and culture time on the aminoacylase activity were shown in Figure 1. Soybean sprout extract medium(A)containing per l: 100 g of soybean,50 g of glucose; Peptone media (B) containing per l: 20 g of glucose,10 g of peptone,1 g of yeast extract,2 g of N-acyl-DLtheanine,2 g of K2HPO4,0.5 g of MgSO4. The pH of the medium was adjusted to 6.5-7.0; Czapack’s media (C) containing per l: 30 g of saccharose,3 g of NaNO3,2 g of N-acyl-DL-theanine,1 g of K2HPO4,0.5 g of KCl,0.5 g of MgSO4·7H2O,10 mg of FeSO4. Pellets cultured 36h on peptone media were obtained the maximum aminoacylase activity.
Figure 1:Effects of media and culture time on the relative aminoacylase activity. The kind of media =▆, Soybean sprout extract medium (A); ●, Peptone media (B); ▲, CzapackÃÆâÃâââ¬Ãâââ¢s media (C). The values were shown as relative activity. The maximum relative activity (R.A.) was indicated as 100%.
3.2 Effect of pH on aminoacylase activity
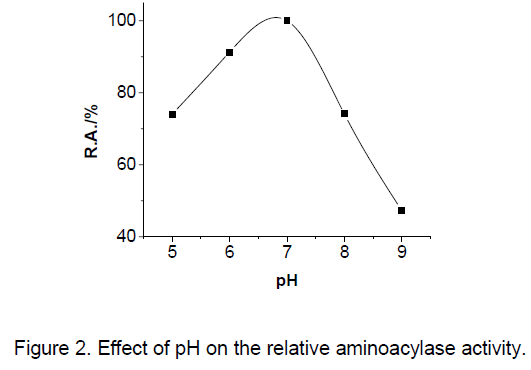
The relative aminoacylase activity plotted against pH could be seen in Figure 2 . Acetate and phosphate buffers were used for 5.0–9.0 pH ranges,respectively. The pH 6.5–7.0 range was optimum for aminoacylase.
3.3 Effect of temperature on aminoacylase activity
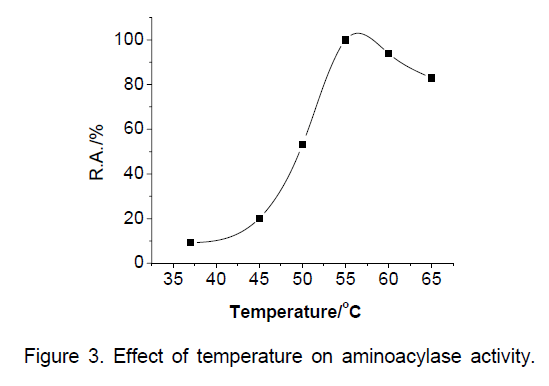
Effect of temperature to the relative activity of the cells were measured over the range of 37-60°C. The results obtained were shown in Figure 3 ,enzymatic activity increased with temperature,reaching a clear maximum value at 55°C. Consequently,the optimal temperature for the production of L-theanine with Laminoacylase of Cunnighamella echinulata 9980 was 55°C.
3.4 Effect of cobalt chloride concentration on aminoacylase activity
It was well known that the enzyme,aminoacylase,obtained from different sources was strongly activated by CoCl2[20],therefore,the effect of CoCl2 concentration,ranging from 0-10-5,on aminoacylase activity of Cunnighamella echinulata 9980 was investigated. As shown in Table 1,the enzymatic activity was improved by the addition of Co2+ to the reaction mixture; the option concentration was 10-3- 10-4. For higher CoCl2 concentrations,an inhibition effect was observed.
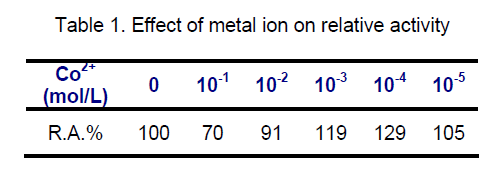
3.5 Effect of buffer concentration on aminoacylase activity
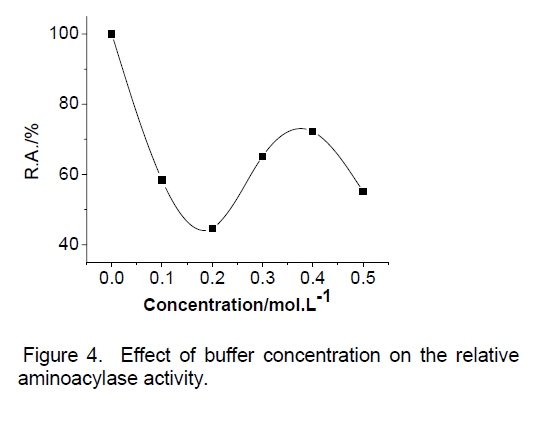
We investigated the effect of buffer concentration,ranging from 0-0.5mol/L,on aminoacylase activity of Cunnighamella echinulata 9980. The maximum activity was obtained at a concentration of 0M. Buffer concentration higher resulted in decreases in the activity measured; the minmum activity was obtained at a conentration of 0.2M phosphate buffer. But when buffer concentration was 0.2M to 0.4M,the activity was a notable increase.
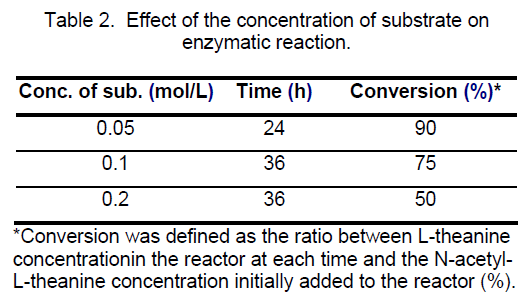
3.6 Effect of initial substrate concentrations on aminoacylase activity
The production of L-theanine in reaction mixture was performed at three initial substrate concentrations: 50,100 and 150 mM. The results obtained were shown in Table 2,the conversion decreased when the substrate concentration increased.
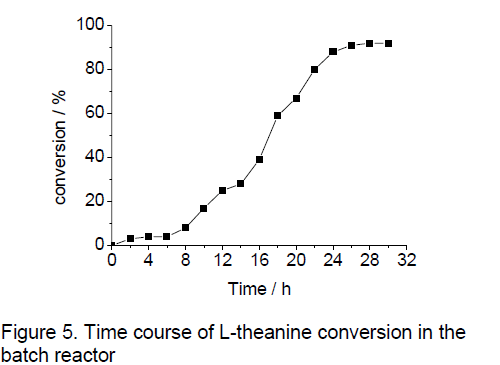
3.7 Time course of L-theanine conversion in reaction mixture
The production of L-theanine in reaction mixture was performed at an initial substrate concentration: 50 mM. The results obtained were shown in Figure 5 ,in which conversion was plotted against time. As expected,the conversion increased with operating time. After 24-h of operation,the maximum conversion obtained,in the tested substrate concentration,was 3.9g.L-1,which was sufficiently high for this solution to be used for further purification steps. Moreover,it was important that no D-theanine was detected in the reaction mixture,so the purity of the L-theanine obtained was 90%.
4 Conclusion
The present study had established a basis for developing an industrial process to produce optically pure L-theanine using Cunnighamella echinulata 9980, a novel aminoacylase-producing fungi. The use of free cells avoided enzyme extraction and purification, which were expensive and time-consuming steps. Operation in a Erlenmeyer flask gave a good result Cunnighamella echinulata 9980 showed very high L-aminoacylase activity. It was therefore possible to anticipate the advantages, which would arise from scaling up.
Acknowledgement
This work is supported by the innovation foundation of china( No. 02CJ-13-01-16).
References
- Sakato Y. (1949) The chemical constituents of tea: III. A new amide theanine,Nippon Nogeikagaku Kaishi. 23: 262-267.
- Goto T.,Horie H.,Ozeki Y.,et al. (1994) Chemical composition of Japanese green tea on market,Tea Res J. 80: 23-28.
- Goto T.,Yoshida Y.,Amano I.,et al. (1996) Chemical composition of commercially available Japanese green tea,Foods& Food Ingred J. 170: 46-51.
- Casimir J.,Jadot J.,Renard M. (1960) Separation and characterization of Nethyl-γ-glutamine in Xerocomus badius (Boletus ladius),Biochim Biophys Acta. 39: 462-468.
- Nakagawa M. (1970) Constituents in tea leaf and their contribution to the taste of green tea liquor. Jpn Agric Res Q. 5: 43-47.
- Yokogoshi H.,Kato Y.,Sagesaka Y.M. (1995) Reduction effect of theanine on blood pressure and brain 5-hydroxyindoles in spontaneously hypertensive rats. Biosci Biotechnol Biochem. 59: 615-618.
- Kimura R.,Murata T. (1971) Influence of alkylamides of glutamic acid and related compounds on the central nervous system. I. Central depressant effect of theanine. Chem Pharm Bull. 19: 1257-1261.
- Kimura R.,Kurita M.,Murata T. (1975) Influence of alkylamides of glutamic acid and related compounds on the central nervous system. III. Effect of theanine on spontaneous activity of mice. Yakugaku Zasshi. 95 892-895.
- Kobayashi K.,Nagato Y.,Aoi N.,et al. (1998) Effects of L-theanine on the release of alpha-brain wave human volunteers. Nippon Nogeikagaku Kais 153-157.
- Sadzuka Y.,Sugiyama T.,Miyagishima A.,et al. (1996) The effects of theanine,as a novel biochemical modulator,on the antitumor activity of adriamycin.Cancer Letters. 105( 2): 203-209.
- Sadzuka Y.,Sugiyama T.,Suzuki T.,et al. (2001) Enhancement of the activity of doxorubicin by inhibitio of glutamate transporter. Toxicology Letters. 123: 159- 167.
- Sugiyama T.,Sadzuka Y.,Tanaka K.,et al. (2001) Inhibition of glutamate transporter by theanine enhances the therapeutic efficacy of doxorubicin Toxicology Letters. 121: 89-96.
- Tachiki T.,Suzuki H.,Wakisaka S.,et al. (1986) Production of γ-glutamylmethylamide and γ- glutamylethylamide by coupling of baker’s yeast preparations and bacterial glutamine synthetase. J Gen Appl Microbiol. 32: 545-548.
- Matsuura T.,Kakuda T. (1990) Effects of precursor,temperature,and illumination on theanine accumuin tea callus. Agric Biol Chem. 54: 2283-2286.
- Orihara T.,Furuya T. (1990) Production of theani and other γ-glutamyl derivatives by Camellia cultured cells. Plant Cell Rep. 9: 65-68.
- Tachiki T.,Yamada T.,Mizuno K.,et al. (1998) γ-Glutamyl transfer reactions by glutaminase from Pseudomonas nitroreducens IFO 12694 and their application for the syntheses of theanine and γ-glutamylmethylamide. Biosci Biotechnol Biochem 62:1279-1283.
- Abelian V.H.,Okubo T.,Shamtsian M.M.,et al.( 1993)A novel method of production of theanine by immobilized Pseudomonas nitroreducens cells. Bios Biotechol Biochem.57:481–483.
- Abelian V.H.,Okubo T.,Mutoh K.,et al.( 1993) A continuous production method for theanine by immobilized Pseudomonas nitroreducens cells. J Ferment Bioeng .76:195–8.
- Juneja L.R.,Chu Djong-Chi,Okubo T.,et al.(1999) Ltheanine-a unique amino acid of green tea and its relaxation effect in humans. Trends in Food Sci.Technol. 10:199-204
- Marshall R.,Birnbaum S.M.,Greenstern J.P. (1956) Cobalt ion activation of renal acylase I. J. Am. Chem.Soc. 78: 4636-4642.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences