Virulence Factors of Shiga-Toxigenic Escherichia coli in Drinking Water of Shahrekord, Iran
Mina Shojaei
Department of Medical Records, Social Security Organization, Chaharmahal Va Bakhtiari Province, Shahrekord, Iran.
Received date: November 10, 2016; Accepted date: November 29, 2016; Published date: December 06, 2016
Citation: Shojaei M. Virulence Factors of Shiga-Toxigenic Escherichia coli in Drinking Water of Shahrekord, Iran. Electronic J Biol, 13:1
Abstract
Background: Shiga toxigenic Escherichia coli are a substantial cause of water-borne diseases. The current research was done to study the microbial quality of the drinking water of Shahrekord with respect to the recognition of virulence factors of Shiga toxigenic Escherichia coli.
Methods and findings: A total of 200 drinking water samples were collected and positive strains were analyzed for presence of STEC strains and virulence factors by PCR technique. Ten out of 200 drinking water samples (5%) were positive for E. coli. Seven samples (70%) were STEC. All of the EHEC strains were simultaneously positive for stx1, eaeA and ehly genes. The prevalence of stx1 and eaeA genes, stx2 and eaeA genes and finally stxs and eaeA genes in the AEEC strains were 50%, 25% and 25%, respectively.
Conclusion: The bases of animal and human intestinal contaminations should be control to decrease the menace of E. coli in drinking water of Shahrekord.
Keywords
Shiga toxigenic Escherichia coli; Drinking water; Shahrekord; Virulence factors.
1. Introduction
In a day, milliards of people use from drinking water. The safety and health of drinking water are major concerns throughout the world. Health risks may arise from consumption of water contaminated with infectious agents, radiological hazards and toxic chemicals. It has been estimated that water-borne diseases were the causative agents of more than two million deaths and four billion cases of diarrhea annually [1]. In keeping with this, there are several reports concerning the contamination of drinking water with dangerous pathogens such as Escherichia coli [2].
E. coli is a gram-negative, flagellated, non-sporulating, facultative anaerobic and rod-shaped bacterium which belongs to Enterobacteriaceae family [2-5]. E. coli is characteristically classified into enterotoxigenic E. coli (ETEC), enteroadherent E. coli (EAEC), enteroinvasive E. coli (EIEC), enterohemorrhagic E. coli (EHEC) and enteropathogenic E. coli (EPEC) [2- 5]. EHEC strains are a portion of Shiga-toxigenic E. coli (STEC) strains. STEC strains are accountable for intensive clinical symptoms like bloody diarrhea, hemorrhagic colitis (HC), gastroenteritis and Hemolytic Uremic Syndrome (HUS) [2-5]. The most substantial genes which are related to STEC infections are intimin protein (eaeA), Shiga toxins (stx1 and stx2) and hemolysin (hlyA) [2-6].
Based on the uncertain epidemiological and microbiological aspects of the drinking water in the Shahrekord, Iran, the current examination was performed to study the incidence of STEC strains and their virulence factors in the eating water samples of Shahrekord, Iran.
2. Materials and Methods
2.1 Samples and E. coli identification
From March 2015 to May 2015, a total of 200 drinking water samples were arbitrarily recovered from the numerous locations of Shahrekord, Iran. Dechlorination of water samples was done using the 0.5 g of sodium thiosulphate which was added to the empty sterile bottles of sampling. To diminish the menaces of contamination, water samples were taken about 30 s after opening of faucet. Water samples were directly transported to the workroom in cooler with ice-packs. All procedures were used to deterrence of cross contamination in the process of sampling.
Typical Total Coliform Multiple-Tube (MPN) Technique was used for inspection of water samples. Tubes with typical growth were subjected to blood and MacConkey agar (Merck, Germany) media and incubated for 24 h at 37°C. Typical colonies of E. coli were transferred to the EMB agar (Merck, Germany) media and incubated at 37°C for 24 h. Typical E. coli bacteria were approved using the biochemical tests introduced by Dehkordi et al. [3].
2.2 Genomic DNA purification and PCR amplification
Typical E. coli colonies were sub-cultured on nutrient agar broth (Merck, Germany) and then incubated 24 h at 37ºC. Then, Genomic DNA was extracted from the bacterial colonies using the DNA extraction and purification kit (Fermentas, Germany) according to the manufacture’s instruction. Concentration of extracted DNA samples have been determined by determining absorbance of DNA at 260 nm using spectrophotometer (SQ 2800, Unico, USA) [7]. Genomic DNA samples of each colony were further approved using 16S rRNA gene-based PCR amplification according to the method described by Woo et al. [8].
2.3 Identification of virulence factors
Set of primers and programs of PCR reactions applied for determination of virulence factors are shown in Table 1 [2-6]. Totally, 10 μl of PCR products were committed on a 2% agarose gel consisting of 0.5 mg/ml of CYBR Green (Fermentas, Germany) at 90 V for 20-30 min. Products were interpreted using ultraviolet illumination. E. coli ATCC 8739 and sterile distilled water were applied as positive and negative controls of PCR reactions.
2.4 Arithmetical examination
Data recovered from all tests were subjected to SPSS/19.0 software and P<0.05 was determined a significance level.
3. Results
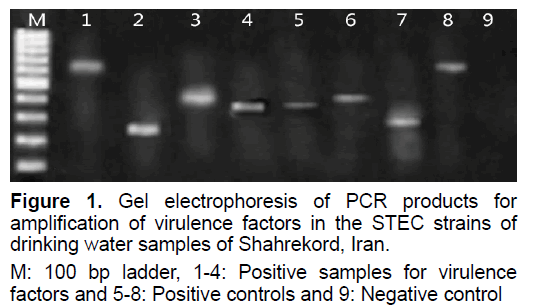
A total of 200 drinking water samples were test for occurrence of STEC strains. Table 2 displayed the incidence of E. coli in the eating water samples of Shahrekord, Iran. Of 200 samples studied, 10 samples (5%) were positive for E. coli. Figure 1 shows the gel electrophoresis of PCR products for determination of virulence genes in the STEC strains of drinking water samples of Shahrekord, Iran. Frequency of virulence genes in the E. coli subtypes isolated from drinking water samples of Shahrekord, Iran is shown in Table 3. Of 10 E. coli strains detected, 7 strains (70%) were STEC. Arithmetical important alteration was perceived amongst the incidence of AEEC and EHEC subtypes (P<0.05). All of the EHEC strains were simultaneously positive for stx1, eaeA and ehly genes. The prevalence of stxs, stx2 and eaeA genes in the AEEC strains were 100%, 25% and 75%, respectively. Besides, the prevalence of stx1 and eaeA genes, stx2 and eaeA genes and finally stx1, stx2 and eaeA genes in the AEEC strains were 50%, 25% and 25%, respectively.
4. Discussion
The current research was focused on the detection of STEC strains and virulence genes in the drinking water samples of Shahrekord, Iran. Totally, 10% of drinking water samples were contaminated with E. coli. Ahmad et al. [9] reported that 26 out of 86 water samples (30.23%) collected from various parts of Pakistan were contaminated with E. coli. Patoll et al. [10] showed that the incidence of E. coli in drinking water samples was 64.29%. Previous study which was performed in Iran showed that 26.38% of tap water and 10.26% of bottled mineral water samples of Isfahan province were positive for E. coli [5]. The prevalence of E. coli in the surface water and waste water samples of Netherland were 88.88% and 100%, respectively [11]. EL-Jakee et al. [12] reported that total prevalence of E. coli in the water samples collected from the sources of agriculture, treated sewage, well, canal, untreated sewage and underground samples were 42.9%, 33.3%, 33.3%, 25, 25% and 7.7%, respectively.
A conceivable cause for the high incidence of E. coli in the drinking water of Shahrekord is due to primary contamination of the Dimeh fountain. Dimeh fountain is the main source of drinking water supply of the Shahrekord. Water of this fountain is transferred through pipelines to the Shahrekord refinery room. Several sources of pollution including primary contamination of Dimeh water, possibility of the linkage of polluted sources like waste waters of factories, cities and agricultural lands through pipes into the Dimeh water and presence of microbial biofilms in the pipelines can contributed in the contamination of drinking water of Shahrekord. Considerable incidence of E. coli in the urban, rural and agricultural waste waters has been reported previously [13,14]. Poor obedience to the principles of water purification and disinfection in the Shahrekord water purification is another cause for the considerable incidence of E. coli in the drinking water samples of this city. Considerable incidence and existence of E. coli biofilm in drinking water supply systems have been reported previously [9,15-17]. Another object for the considerable incidence of E. coli in Shahrekord is that it has open water accumulation sources. Therefore, there were the high possibilities for entrance of foreign material, dust, animals, birds and even human waste into these open water accumulation sources.
Additional significant result of our examination is the considerable incidence of stx1, stx2, eaeA and ehly virulence markers in the E. coli strains of drinking water samples of Shahrekord. The most frequent virulence markers were stx1 and eaeA. In a study which was conducted on Egypt, the stx1 and eaeA genes were detected in the numerous samples of drinking water [18]. Ram et al. [19] reported the occurrence of different types of virulence markers of E. coli from water samples. Total prevalence of stx genes in the E. coli isolates of Dhaka city of Bangladesh was 60% [20]. In a study which was conducted on South Africa, the prevalence of eaeA and stx genes in the Plankenburg and Berg River systems were 15% and 0% and 8% and 15%, respectively [21]. Our results showed that all of the EHEC strains and majority of the AEEC strains harbored the stx1 and eae genes. Considerable incidence of these factors in the water samples of Shahrekord city exposed an imperative community health matter.
Our discoveries are in contract with a beforehand recorded results of expressively advanced occurrence of the eaeA gene (96%) in surface water [22,23]. EHEC strains causes HC and HUS in humans, and main virulence marker consist of eaeA and Shiga toxins genes. The moderately considerable incidence of the stx1 gene compared to stx2 in the E. coli isolates of drinking water recommends that E. coli strains which harbored mixture of the stx1 and eaeA genes is more routine than the mixture of eaeA and stx2 genes. Such of our E. coli isolates had all stx1, stx2 and eaeA genes which increased the importance of matter. The standing of these information lies on the point that eae-positive STEC strains are recognized more virulent for humans than eae-negative one [24]. It has been documented that combination of several virulence markers in such strains of E. coli may cause more sever diseases in humans [25,26].
5. Conclusion
In conclusion, we recognized a large proportion of E. coli bacteria with distinct pathotypes which derived mostly from bases of animal and human in drinking water samples of Shahrekord. We also recognized numerous E. coli strains positive for stx1 and eaeA genes. Concurrent attendance of stx1 and eaeA and stx2 and eaeA virulence markers in such E. coli strains specified the imperative community health concern regarding consumption of water. Application of bacteriological examination can diminish the bacterial load of drinking water. Monitoring of the bases of human and animal intestinal contamination, including management of municipal, industrial and agricultural wastewater is additional method to decrease the occurrence of E. coli in drinking water. It is essential to study the role of biofilms in survival of pathogenic bacteria in water pipes of Shahrekord.
6. Acknowledgement
The authors thank Dr. Ebrahim Rahimi at the Department of Food Hygiene and Quality Control, Faculty of Veterinary Medicine, Shahrekord Branch, Shahrekord, Iran for providing clinical isolates and positive controls of E. coli. Author would also thank from the staffs of the Food Hygiene Research Center of the Islamic Azad University of Shahrekord Branch, Shahrekord, Iran.
7. Funding
This work was supported by the Islamic Azad University of Shahrekord (2015ef744).
References
- WHO. (2000). Water Supply and Sanitation Council. Global Water Supply and Sanitation Assessment 2000 Report. World Health Organization, New York.
- Momtaz H, Dehkordi SF, Rahimi E, et al. (2013). Incidence of Shiga toxin-producing Escherichia coli serogroups in ruminant's meat. Meat Sci. 95: 381-388.
- Dehkordi FS, Yazdani F, Mozafari J, et al. (2014) Virulence factors, serogroups and antimicrobial resistance properties of Escherichia coli strains in fermented dairy products. BMC Res Notes. 7: 217.
- Momtaz H, Dehkordi SF, Hosseini MJ, et al. (2013). Serogroups, virulence genes and antibiotic resistance in Shiga toxin-producing Escherichia coli isolated from diarrheic and non-diarrheic pediatric patients in Iran. Gut Pathog. 5: 39.
- Momtaz H, Dehkordi SF, Rahimi E, et al. (2013). Detection of Escherichia coli, Salmonella species and Vibrio cholerae in tap water and bottled drinking water in Isfahan, Iran. BMC Publ Health. 13: 556.
- Momtaz H, Farzan R, Rahimi E, et al. (2012). Molecular characterization of Shiga toxin-producing Escherichia coli isolated from ruminant and donkey raw milk samples and traditional dairy products in Iran. Sci World J. 2012: 231342.
- Sambrook J, Russell D. (2001). Molecular cloning, a laboratory manual. In Cold Spring Harbor Laboratory. 3rd edition. Cold Spring Harbor, New York.
- Woo PCY, Cheung EYL, Leung K, et al. (2001). Identification by 16S ribosomal RNA gene sequencing of an Enterobacteriaceae species with ambiguous biochemical profile from a renal transplant recipient. Diagn Microbiol Infect Dis. 39: 85ÃÆâÃâââ¬Ãâââ¬Å93.
- Ahmad MD, Hashmi RA, Anjum AA, et al. (2009). Drinking water quality by the use of congo red medium to differentiate between pathogenic and non-pathogenic E. coli at poultry farms. J Animal Plant Sci. 19: 108-110.
- Patoli AA, Patoli BB, Mehraj V. (2010). High prevalence of multi-drug resistant Escherichia coli in drinking water samples from Hyderabad. Gomal J Med Sci. 8: 23-26.
- Heijnen L, Medema G. (2006). Quantitative detection of E. coli, E. coli O157 and other shiga toxin producing E. coli in water samples using a culture method combined with real-time PCR. J Water Health. 4: 487-498.
- EL-Jakee J, Moussa EI, Mohamed KHF, et al. (2009). Using molecular techniques for characterization of Escherichia coli isolated from water sources in Egypt. Global Vet. 3: 354-362.
- Chern EC, Tsai YL, Olson BH. (2004). Occurrence of genes associated with enterotoxigenic and enterohemorrhagic Escherichia coli in agricultural waste lagoons. Appl Environ Microbiol. 70: 356-362.
- Oliver JD, Dagher M, Linden K. (2005). Induction of Escherichia coli and Salmonella typhimurium into the viable but non-culturable state following chlorination of wastewater. J Water Health. 3: 249-257.
- Juhna T, Birzniece D, Rubulis J. (2007). Effect of phosphorus on survival of Escherichia coli in drinking water biofilms. Appl Environ Microbiol. 73: 3755-3758.
- Yu J, Kim D, Lee T. (2010). Microbial diversity in biofilms on water distribution pipes of different materials. Water Sci Technol. 61: 163-171
- Ryu J, Beuchat LR. (2005). Biofilm formation by Escherichia coli O157:H7 on stainless steel: Effect of exopolysaccharide and curli production on its resistance to chlorine. Appl Environ Microbiol. 71: 247-254.
- Galal HM, Hakim AS, Dorgham SM. (2013). Phenotypic and virulence genes screening of Escherichia coli strains isolated from different sources in delta Egypt. Life Sci J. 10: 352-361.
- Ram S, Shanker R. (2005). Computing taq Man probes for multiplex PCR detection of Escherichia coli O157 serotypes in water. Silico Biol. 5: 1-6.
- Munshi SK, Rahman MM, Noor R. (2012). Detection of virulence potential of diarrhoeagenic Escherichia coli isolated from surface water of rivers surrounding Dhaka city. J Bangladesh Acad Sci. 36: 109-121.
- Sidhu JP, Ahmed W, Hodgers L, et al. (2013). Occurrence of virulence genes associated with diarrheagenic pathotypes in Escherichia coli isolates from surface water. Appl Environ Microbiol. 79: 328-335.
- Masters N, Wiegand A, Ahmed W, et al. (2011). Escherichia coli virulence genes profile of surface waters as an indicator of water quality. Water Res. 45: 6321-6333.
- Shelton DR, Karns JS, Higgins JA, et al. (2006). Impact of microbial diversity on rapid detection of enterohemorrhagic Escherichia coli in surface waters. FEMS Microbiol Lett. 261: 95-101.
- Willshaw GA, Scotland SM, Smith HR, et al. (1994). Hybridization of strains of Escherichia coli O157 with probes derived from the eaeA gene of enteropathogenic E. coli and the eaeA homolog from a Vero cytotoxin-producing strain of E. coli O157. J Clin Microbiol. 32: 897-902.
- Boerlin P, McEwen SA, Boerlin-Petzold F, et al. (1999). Associations between virulence factors of Shiga toxin producing Escherichia coli and disease in humans. J Clin Microbiol. 37: 497-503.
- Paton AW, Paton JC. (1998). Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol. 36: 598ÃÆâÃâââ¬Ãâââ¬Å602.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences