The Physiological Mechanism of Populus euphratica Adapting to Drought Stress
Yongqing Yang, Wenqi Wang, Eric A. Ottow, Andrea Polle, Fuchun Zhang, Haiyan Lan, Xiangning Jiang
Yongqing Yang1,2,Wenqi Wang1,Eric A. Ottow3,Andrea Polle4,Fuchun Zhang4,Haiyan Lan4,Xiangning Jiang1,*
1College of Life Sciences and Biotechnology,Beijing Forestry University,Beijing 100083,China
2National Institute of Biological Sciences,Beijing 102206,China
3Poplar Research Group,Institute for Forest Botany,University of Göttingen,Büsgenweg 2,37077 Göttingen,Germany
4National key laboratory Basis of Xinjiang Biological Resources and Genetic Engineering,College of Life Sciences,Xinjiang University,Urumuqi,Xinjiang 840034,China
Abstract
P. euphratica is well known for its high drought tolerance. It is the few tree species that forms natural forests in the semiarid area, which mainly distributes in Takla Makan Desert of Xinjiang in China. In order to study the mechanism of drought tolerance of P. euphratica, we investigated, sampled and analyzed the trees in Takla Makan Desert of Xinjiang. The results showed that the stomatic resistance increased as well as transpiration decreased in leaves of P. euphratica under drought stress. Compared with control, under serious drought stress the contents of K+ and Ca2+ increased 0.43 and 1.99 times, respectively, but there is no change in the contents of Mg2+ and Na+. The proton-pumping activities of plasma membrane bound H+-ATPases hardly differed between leaves from control and middle drought stress grown P. euphratica plants. However, under serious drought stress the proton-pumping activities decreased. These results indicating that P. euphratica adapts to drought stress by various way, such as decreasing transpiration, absorbing ion selectively, reducing ATP consuming.
Keywords
Populus euphratica,Drought stress,Plasma membrane H+-ATPase,Transpiration,Ion contents
1. Introduction
Drought was a mainly problem to the plants,more than 1/3 of lands are affected worldwide by drought stress. The limitation of growth and productivity of most plant species by drought outweighs other environmental stresses [1]. There was about 2/3 arid and semiarid area in China,especially in northwest,drought was a main factor that influenced the growth of forestry. Furthermore,the desert spread was speeded by drought,about 3.857×105 km2 salt-affected soils in north China [2].
Euphratica,one of the oldest species of Populus in Salicaceae,is the sole species of the genus naturally growing in desert. Its distribution ranges from the northern Africa,Middle East to the northwest of China [3,4]. The total area of P. euphratica forestry is about 64.87×104 hm2 in world,90% of which distributes in China and middle part of Asia [5]. Distribution of P. euphratica is mainly in Takla Makan Desert of Xinjiang in China [6,7]. Severe environmental stresses in these semiarid areas,mainly drought and salinity,limit growth and productivity of most plant species [8].
Chen et al found that P. euphratica increased the proline content under drought stress [9]. Although P. euphratica showed the ability for drought tolerance,but its growth is correlated to environmental water condition [10]. P. euphratica displayed a rise of ABA content with the decrease of water table [11]. In suspension culture,compared with control (without PEG) the growth of P. euphratica cell showed no change in the medium containing 25% (W/W) PEG indicating its ability for drought stress [4]. The physiological background underlying drought tolerance of P. euphratica is still poorly understood. The aim of this study was,to gain new information about putative strategies to cope with drought stress. In the present study we investigated changes in plasma membrane H+-ATPase activity,transpiration and various ions content in P. euphratica in Takla Makan Desert of Xinjiang in China in response to drought stress.
2. Materials and Methods
2.1. Plant materials
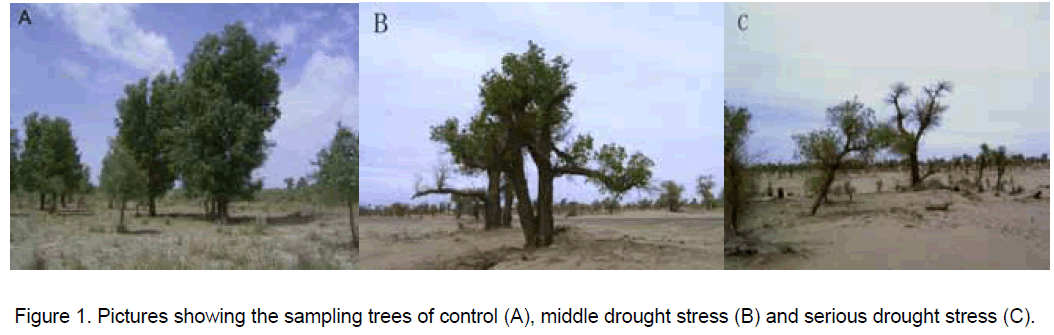
Mature P. euphratica trees (Figure 1),naturally growing along the Takla Makan Desert of Xinjiang in China,control (41O10'19"N,84O14'03"E,914 m elevation),middle stress (40O58'55"N,84O14'42"E,917 m elevation) and serious stress samples (40O46'31"N,88O17'33"E,923 m elevation) were sampled in May 2005. The sampled plants were 6-8 m in height,healthy and free of infections. Leaves were harvested within four different distances from the ground,placed in pre-wetted cotton gauze and subsequently placed on ice and stored at -80 °C until further analysis.
2.2 Transpiration and stomatic resistance measurement
The transpiration of P. euphratica leaves was measured using Li-1600 apparatus. The sample trees were described as above.
2.3 Leaves ion contents measurement
Ions content was quantitated using AAS (Shimadzu,Kyoto,Japan). The collected 0.5g leaves were added to 5 ml of a HNO3 / HClO4 (5:1) solution. Afterwards,they were heated at 300 °C until white smoke got visible. Finally,the digestion solution was diluted in 5 ml of distilled water for ions measurement.
2.4 Plasma membrane isolation and purification
Plasma membrane vesicles were prepared from the P. euphratica leaves using aqueous two-phase partitioning according to the method described by Palmgren et al. [12] and Larsson et al. [13]. All procedures were carried out at 4°C. The resulting plasma membrane pellets were immediately frozen in liquid nitrogen and stored at –80°C.
2.5 Plasma membrane H+-ATPase hydrolytic and proton pumping activity
ATP hydrolytic and proton pumping assays were carried as described in Yan et al. [14].
2.6 Protein quantification
Protein content was determined by the dye-binding method of Bradford [15] using bovine serum albumin as a standard.
2.7 Data analysis
Significant differences between samples were calculated by using the Student’s t test. Variation is indicated by ± SD.
3. Results and Discussion
3.1 Description of sampling area
The study areas belong to the warm temperate zone displaying a dry continental climate. The mean annual precipitation is 65.6 mm while the annual potential evaporation is 2024 mm. The air temperature has large daily fluctuations with an annual maximum temperature of 41 °C and the minimum being as low as –25 °C. The northwest blows frequently throughout the entire year. The relative air humidity usually does not exceed 50%. From above pictures we can seen that there are many kinds of plant species existing in the control sampling area,but few other plants in the middle and serious drought stress area,indicating P. euphratica is a relative drought tolerant plant.
3.2 Transpiration and stomatic resistance analysis
Pore plays an important role in the plant metabolism,such as photosynthesis and transpiration [16]. The factors including light intensity,transpiration and soil water,especially soil water content which affect the transpiration [17].
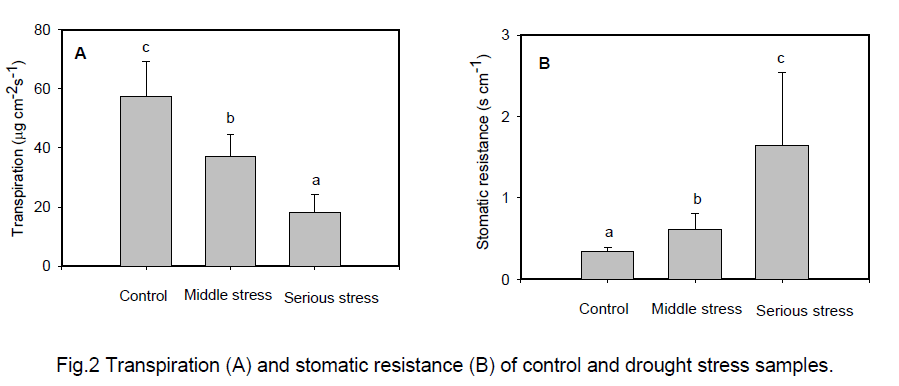
In the present study we investigated the changes of transpiration and stomatic resistance of P. euphratica to cope with drought stress. Our results demonstrate that under drought stress stomatic resistance increased (Figure 2B) as well as transpiration decreased (Figure 2 A) in leaves of P. euphratica. Obviously,P. euphratica can adapt drought stress via adjusting the stomatic resistance and reducing water loss.
3.3 Effects of drought stress on the ion contents of P. euphratica leaves
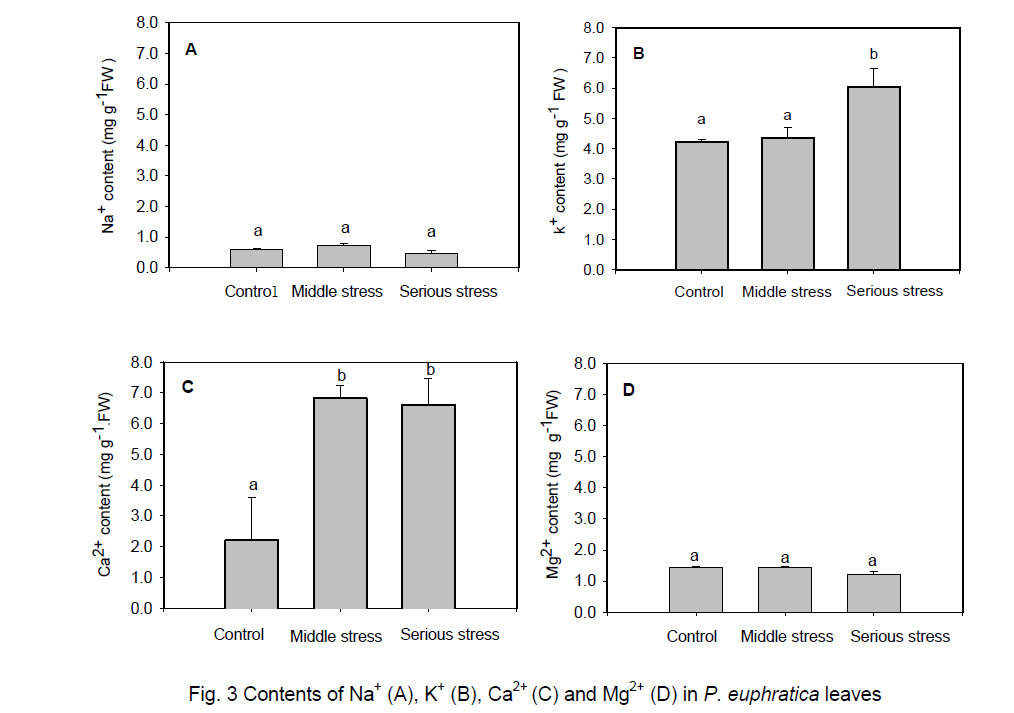
An additional osmosis stress was imposed upon the P. euphratica trees under drought stress. To prevent osmosis stress,inorganic ions or organic compound are accumulated in plants [18-20]. In order to study the effect of drought stress on the ions content,we investigated several major ions between control and drought stressed P. euphratica leaves. No difference of Na+ and Mg2+ could be measured in the control and salt-alkali stress leaves (Figure 3 A,D). When exposed to serious drought stress conditions,a slight increase of K+ was observed in the P. euphratica leaves (Figure 3 B). In contrast,there was an obvious increase of Ca2+ content under middle and serious drought stress conditions (Figure 3 C). These results demonstrate that some kinds of ions can be absorbed selectively in P. euphratica leaves under drought stress .
3.4 Plasma membrane vesicles purity examination
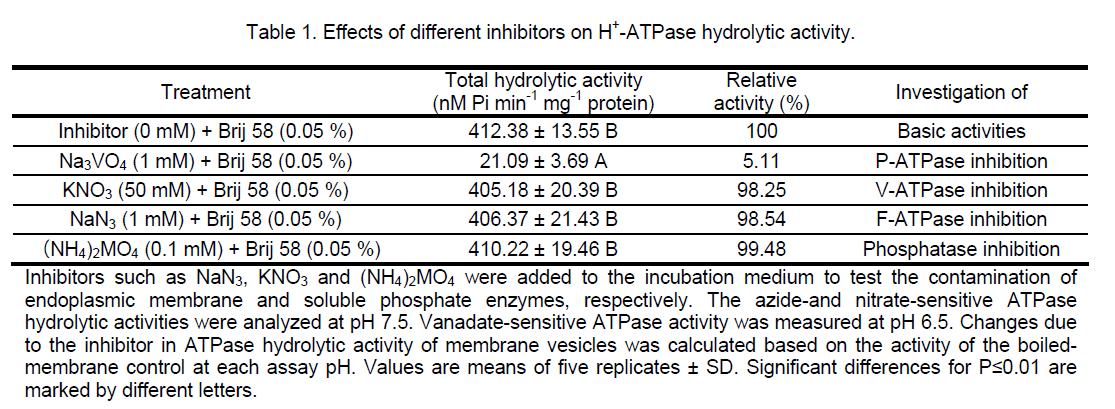
For H+-ATPase assay,plasma membrane vesicles were isolated and purified from P. euphratica leaves using aqueous two-phase partitioning. To examine the purity of these vesicles preparations,the H+-ATPase hydrolytic activity was assayed in the absence and presence of a number of inhibitors. As can be seen in Table 1,the H+-ATPase hydrolytic activity was reduced by about 95% in the presence of 1 mM vanadate (Na3VO4,an inhibitor of the plasma membrane H+-ATPase). On the other hand,the H+-ATPase hydrolytic activity showed anegligible sensitivity to azide (NaN3,an inhibitor of mitochondrial ATPase),nitrate (KNO3,an inhibitor of vacuolar ATPases) and molybdate ((NH4)2MO4,an inhibitor of nonspecific phosphatases). These results demonstrate that preparations are enriched in plasma membranes vesicles without any significant contamination from other cellular membranes.
3.5 Plasma membrane H+-ATPase hydrolytic activity
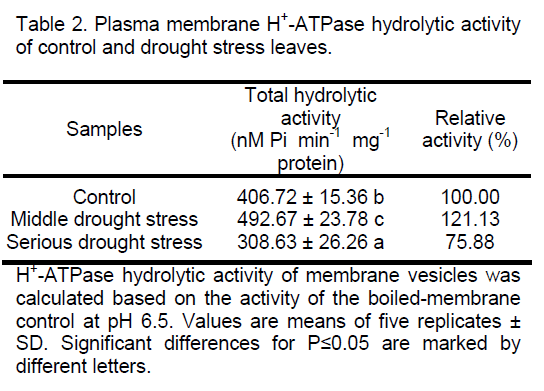
Previous studies showed that the plasma membrane H+-ATPase activity was decreased under water stress [21]. Gong et al. [22] reported that the H+-ATPase activity was reduced in leaves of wheat under drought stress. The activity was changed in the callus of wheat after PEG6000 treatment [23]. The plasma membrane H+-ATPase acts as a primary transporter hydrolyzing ATP and pumping protons out of the cell. Changes of hydrolytic activities in control and drought stress P. euphratica leaves were investigated. Results from our experiments showed that the plasma membrane H+-ATPase hydrolytic activity was increased by about 21% in the middle drought stress leaves,but decreased about 25% under serious drought stress (Table 2).
3.4 Plasma membrane H+-ATPase proton pumping activity
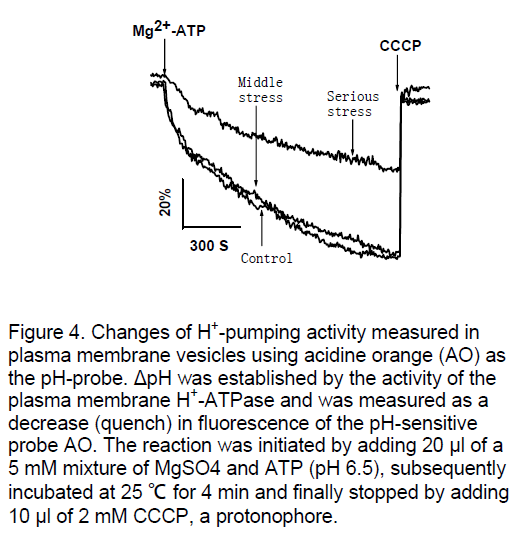
H+-pumping activity of these membrane vesicles was characterized to demonstrate their proton transport competence. In Figure 4 ,after initiation of H+-pumping by adding Mg2+ and ATP,there was a rapid quenching in the vesicles. As expected,this established pH gradient completely collapsed after addition of the protonphore CCCP. Nearly no difference was observed between the control and middle drought stress leaves vesicles,but decreased obviously under serious drought stress.
Figure 4. Changes of H+-pumping activity measured in plasma membrane vesicles using acidine orange (AO) as the pH-probe. ΔpH was established by the activity of the plasma membrane H+-ATPase and was measured as a decrease (quench) in fluorescence of the pH-sensitive probe AO. The reaction was initiated by adding 20 μl of a 5 mM mixture of MgSO4 and ATP (pH 6.5), subsequently incubated at 25 °C for 4 min and finally stopped by adding 10 μl of 2 mM CCCP, a protonophore.
4. Conclusion
The physiology mechanism of P. euphratica responses against drought stress can be found that are defined by a range of adaptations. In the present study we investigated changes of some physiologies in P. euphratica leaves in response to drought stress. We show that P. euphratica adapts to drought stress can by decreasing transpiration,absorbing ion selectively,reducing ATP consuming.
Acknowledgements
The work was jointly supported by the National Grand Basic Fundamental Research “973” Project (G1999016005) to Dr. Xiangning Jiang and National Natural Science Foundation Project (Grant No.30271066) to Xuemei Chen. We are grateful to the German Academic Exchange Service (DAAD) project of Dr. Eric A. Ottow (University of Goettingen University,Germany) and BLE funding to Professor Dr. X. J. Zhang (Institute of Biophysics,Chinese Academy of Sciences,P. R. China) for ATPase assay assistance,to Mrs. J. C. Zhou and R. G. Wang (Beijing Forestry University,P. R. China) for providing AAS Na+ determination,to P. Y. Huang (Xinjiang University,P. R. China) for investigation of P. euphratica.
References
- Li J.Y.,Zhai H.B. (2000) Hydraulic architecture and drought resistance of woody plants. Chinese Journal of Applied Ecology,11 (2): 301-305.
- Wang T.,Zhu Z. D.,Wu W. (2002) Sanddy desertification in the north China. Science in China (Series D),45 (Supp.): 23-34.
- Browicz K. (1977) Chorology of Populus euphratica Olivier. Arbor Kórnickie,22: 5-27.
- Gu R.S.,Liu Q.L.,Pei D.,et al. (2004) Understanding saline and osmotic tolerance of Populus euphratica suspended cellsÃÆïÃâüÃâà ½Plant Cell,Tissue and Organ Culture,78(3): 261-265.
- WANG S J,CHEN B H. Forestry of Populus euphratica M. China environmental science publishing house,1995: 15-28.
- Wang S.J. (1996) The actuality of Populus euphratica tree and the countermeasure of conservation and restoration. World forestry research,9(6): 37-43.
- Li Z.Z.,Liu J.P.,Yu J.,et al. (2003) Investigation on the characteristics of biology and ecology of Populus euphratica and Populus pruinosa J. Acta Bot. Boreal.-Occident. Sin.,23 (7): 1292-1296.
- BOYER J S. Plant productivity and environment J. Science,1982,218: 443-448.
- Chen Y. P.,Chen Y.N.,Li W.H.,et al. (2003) Analysis on the change of drought stress on praline of Populus euphratica in the lower reaches of Tarim River. Arid land geography,26(4): 420-424.
- Si J.H.,Feng Q.,Zhang X.Y. (2005) Leaf water potential of Populus euphratica and influencinge factors in extreme arid region. Journal of desert research,25 (4): 505-510.
- Chen Y.P.,Chen Y.N.,Li W.H.,et al. (2004) Analysis on the physiological characteristic of Populus euphratica under drought stress in the lower reaches of Tarim River. Acta Bot. Boreal.-Occident. Sin.,24 (10): 1943-1948.
- Palmgren M.G.,Askerlund P,Fredrikson K,et al. (1990) Sealed inside-out and right-side-out plant plasma membrane vesicles. Optimal conditions for formation and separationÃÆïÃâüÃâà ½Plant Physiol.,92(4): 871-880.
- Larssson C.,Sommarin M.,Widelli S. (1994) Isolation of highly purified plant plasma membranes and the separation of inside-out and right-side-out vesicles. Meth. Enzymol.,228(11): 451-469.
- Yan F.,Zhu Y.Y.,Müller C.,et al. (2002) Adaptation of H+-pumping and plasma membrane H+-ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol.,129(1): 50-63.
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.,72: 248-254.
- Zheng Y.F.,Yan J. Y.,Zhang W. G. (1995) Response of wheat stomatic resistance on weather conditions. Chin. Agron. Met.,16 (3): 9-13.
- Zhang X.Y.,Meng K.,Sui Y.Y. (1999) Change of stomatal resistance in soybean canopy at deferent nutritional levels. System Sciences and Comprehensives in Agriculture,15(4): 302-305.
- Garg A.K.,Kim J.K.,Owens T.G.,et al. (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stressesÃÆïÃâüÃâà ½Proc. Acad. Sci. USA,99(25): 15898 -15903.
- Taji T.,Oshumi C.,Luchi S.,et al. (2002) Important roles of drought- and cold inducible genes for galactinol synthase in stress tolerance in Arabidopsis thalianaÃÆïÃâüÃâà ½Plant J.,29(4): 417-426.
- Ma W.Y. (2004) Progress in research of plant tolerance to Saline Stress. Agriculture and technology,24(4): 95-99.
- Mao G. L.,Xu X.,Xu Z.Z. (2003) Plant plasma membrane H+-ATPase and its response to stresses. Journal of ningxia agricultural college,24(4): 81-91.
- Gong H.J.,Chen K.M.,Chen G.C.,et al. (2003) Effects of gradual drought on the fatty acid composition of polar lipids,H+-ATPase and 5’-AMPase activities in the plasma membrane of two spring wheat leaves. Acta. phtoecologica sinica,27(4): 459-465.
- Shao Y.J.,Li G.M.,Huang G.C.,et al. (2000) Effect of water stress on the activity of PM-ATPase and 5’-AMPase of common winter wheat. Agriculture research in the arid areas,18 (3): 93-97.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences