Salivary Cortisol, Testosterone and DHEA in Healthy University Male Students with Hyperleptinemia: Retrospective Cohort Study
Mahmoud Abu-Samak, Luai Hasoun, May Ibrahim Abu-Taha, Shady Helmi Awwad, Beisan Ali Mohammad
Mahmoud Abu-Samak1,*, Luai Hasoun1, May Ibrahim Abu-Taha1, Shady Helmi Awwad2, Beisan Ali Mohammad3
1Department of Clinical Pharmacy and Therapeutics Applied Science Private University, Amman, Jordan
2Department of Pharmaceutical Chemistry and Pharmacognosy, Applied Science Private University, Amman, Jordan
3Department of Physiological Sciences, Fakeeh College for Medical Sciences, Jeddah, Kingdom of Saudi Arabia (BAM)
Received date: March 06, 2018; Accepted date: March 20, 2018; Published date: March 30, 2018
Citation: Abu-Samak M, Hasoun L, Abu-Taha MI, et al. Salivary Cortisol, Testosterone and DHEA in Healthy University Male Students with Hyperleptinemia: Retrospective Cohort Study. Electronic J Biol, 14:1
Abstract
Background: Cortisol, testosterone and DHEA are steroid hormones associated with youth physiology. The aim of this study was to evaluate the association of stress hormones with leptin, the hormone of obesity, in university male students. In this prospective cohort study, ninety university male students in the age range of 18 to 24 years were categorized into two groups; NL=Normal or Borderline Serum Leptin Level (<9.4 ng/ml); HL=High Serum Leptin Level (≥ 9.4 ng/ ml). In these groups, serum leptin and salivary levels for testosterone, dehydroepiendisterone (DHEA) and cortisol were immunoassayed. Correlation coefficients model analysis found that M-DHEA was significantly positively correlated with serum leptin in all subjects regardless of study group (r=- 0.248, p=0.003). The model analysis also found that body weight showed greater significant and positive correlation with serum leptin level than did BMI (NL: r=0.549 vs. 0.429, HL; r=0.517 vs. 0.422, respectively). A positive correlation between hyperlipidemia and hyperleptinemia was noted. Negative correlation was seen between M-DHEA and some obesity parameter including BMI and body weight and serum leptin. Elevated cortisol with declined DHEA balance was noted in this study. Our findings shed some light on the potential mechanisms linking obesity, stress and aging hormones in young men where leptin is the main obesity marker that promotes steroid hormones imbalance leading to maladaptation to chronic stress in young men. We observed that serum leptin levels are proportional to DHEA/cortisol imbalance and suggested to be a new indicator of chronic stress maladaptation in young obese males.
Keywords
Salivary hormone; Testosterone; DHEA; Cortisol; Leptin; Obesity; Youth.
Introduction
Obesity and stress in youth are a growing concern.
The prevalence of overweight and obesity among Jordanians young males has increased in the past 10 years [1]. Complications of youth overweight and obesity are well-documented and include metabolic health risk, chronic diseases, psychosocial problems [2] and an increased risk of cardiovascular diseases in adulthood [3]. Serum and salivary levels of steroid hormones have been linked with the obesogenic changes in eating behaviors, body fat distribution and metabolism [4]. Due to complex interaction between steroid hormonal activities during youth, identifying a unique clinical strategy to counteract this kind of change remains an existent challenge.
To some extent, steroid hormones are independent of each other particularly among older adolescents than younger adolescents [5]. Remarkable association of elevated salivary or serum cortisol levels in response to acute stress have been linked with weight gain in several human studies [6]. Furthermore, in animal models, high corticosterone responses to adrenocorticotropin releasing hormone (ACTH) has augmented the risk for diet-induced obesity [7].
Obesity hormone, leptin, is now believed to be the leading cause of fat gain in humans [8]. It is more reliable than body mass index as an obesity marker [9]. Human cohort studies show a positive correlation between serum levels of cortisol and leptin [10]. In clinical trials, subjects with Cushing’s disease display consistently elevated leptin levels, which tend to gradually decrease after treatment [11]. Even so, some of related studies concluded that dynamic circadian rhythm of leptin is generally inverse to cortisol, suggesting counter-regulation or ‘antagonism’ [12,13]. However, evidence linking various indices of cortisol and obesity marker in humans is inconsistent at all [14]. Contrary to cortisol, another steroid hormone Dehydroepiandrosterone (DHEA) is described as the ‘elixir of youth’ for its antiaging properties and anti-obesity effects [15]. DHEA replacement may decrease serum leptin levels [16].
An antagonistic relationship between DHEA and cortisol has been described previously [17]. Recently [18] it has been concluded that cortisol/DHEAS ratio may be more informative than the effect of cortisol and DHEAS levels separately.
DHEA reduces circulating levels of cortisol [19] and it is inversely related to peak cortisol in response to CRH infusion [20] and therefore, it antagonizes neurotoxic effects of cortisol [21]. Although these findings may indicate a stress-buffering effect of DHEA, it is reported that salivary cortisol was positively associated with left hippocampus activity [22]. Nevertheless, not all previous studies have approved a reciprocal relationship between DHEA and cortisol [23]. DHEA/cortisol imbalance with the individual's age that reflects stress maladaptation remains a debatable point and needs to be clarified [24,25]. Accordingly, it seems that DHEA/cortisol imbalance is driven by the influence of several stress related factors, including adiposity, rather than the effect of age as a single factor. Serum leptin as an adiposity marker may explain the variation of the cortisol/DHEA balance as seen before in a cross sectional study conducted on young males with allergic rhinitis as a stress model [26].
The principal male sex steroid hormone, testosterone (T), has also been considered as an age and an obesity dependent steroid hormone [27]. For example, less T was secreted by middle-aged men at night compared to healthy young men [28]. Further, low T levels showed inverse effect on metabolic and body compositional parameters, including increases of overall adiposity [14] and visceral fat accumulation [29]. Testosterone is therefore susceptible to the effect of cortisol/DHEA balance. We hypothesize that DHEA/cortisol balance in university male students will be affected by youth obesity rather than the single effect of an individual's age. Thus we aim to estimate the correlation between serum levels of leptin and salivary levels of steroid hormones in university male students.
Research Design and Methods
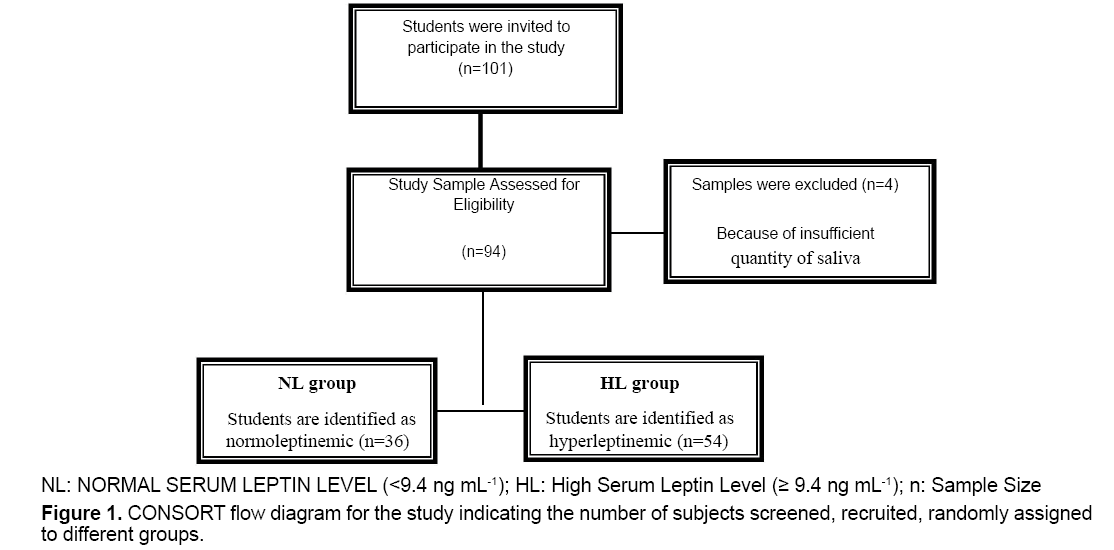
This retrospective cohort study carried out in the Applied Science Private University, Amman, Jordan during the period from March to May 2008. To avoid some anticipated variations in the study sample, only Jordanian students who live in Amman participated in the study. The participants filled out a questionnaire including anthropometric and clinical parameters. From one hundred and one (101), ninety four (94) healthy nursing male students with age between 18 and 24 years were invited to participate in this study and their samples were taken for analysis. Seven students were excluded because they did not complete the questionnaire information. Based on fasting serum levels of leptin, participants were categorized into two groups, a normoleptinemic (NL) group with serum leptin levels <9.4 ng/mL and a hyperleptinemic (HL) group with serum leptin levels ≥ 9.4 ng/mL [30] (Figure 1).
Informed consent and health screening
This study was performed using a protocol no. DRGS-2007-7, approved by the ASU ethics committee for the protection of human subjects. The study was conducted in accordance with the Helsinki Declaration. All participants were provided with an information sheet, which contained details of the experimental protocol. Participants fully understood the purpose of the study as well as the risks involved. Participants were informed of being free to withdraw from the investigation at any stage. All participants provided written consent prior to the commencement of the study and were asked to complete a health screening questionnaire prior to their participation in the study. To avoid confounding factors known to affect leptin and salivary steroid hormones (SSH): Cortisol (C), Testosterone (T) and Dehydroepiendosterone (DHEA) levels, participants with diagnosed chronic diseases such as CVDs, diabetes, hepatic, renal, or endocrine disorders and those who had been taking any kind of medication for the past two months prior to the study were excluded.
Serum glucose and lipid profile
Fasting blood samples were obtained, centrifuged, and stored at -20°C until being assayed. Fasting blood glucose samples were collected at 8 a.m. Blood glucose levels were measured using One Touch® test strips (LifeScan; Johnson & Johnson, Palmitas, CA, USA). Triglycerides, total cholesterol and high-density lipoprotein cholesterol (HDL-C) were determined using enzymatic colorimetric kits (Linear Chemicals, Barcelona, Spain). Low-density lipoprotein cholesterol (LDL-C) was calculated from the equation recorded in previous studies [31].
Salivary steroid hormones (cortisol, testosterone and DHEA)
Salivary steroid hormones (SSH) have been widely used as an indicator of the hypothalamic-pituitarygonadal (HPG) and hypothalamic-pituitary-adrenal (HPA) axes activity. SSH were collected from the participants between 8 and 9 am in the morning (M) for the measurement of the three hormones; C, T and DHEA, and between 11 and 12 pm at night (N) only for C and DHEA. To collect salivary samples, participants were provided with a Salivette® sampling device (cotton) along with both verbal and written instructions for usage. The instructions stated that participants were to collect saliva by themselves. Participants were asked to drool passively through a straw into a tube, which was then kept on ice to precipitate mucins and then centrifuged (10,000x g, 15 min, 4°C). The supernatant (1 mL) was collected and stored at -20ºC until assayed 2 weeks later at Ibn Al-Haytham Hospital laboratories in Amman, Jordan. SSH samples for C, T and DHEA were measured using an enzyme-linked immunosorbent assay (ELISA), SLV-2930, SLV-3013, SLV-3012, respectively (DRG International, Inc., USA). The limits of detection of this assay were 1.48 pmol/ L for C, 1.9 pmol/L for T and 0.324 pmol/ L for DHEA at a 95% confidence limit. The normal range for sT is between 21.2 and 801.2 pmol/L.
Serum Leptin
Fasting serum leptin samples were liquated and stored in polypropylene vials at -20°C until being analyzed 2 weeks later at Al-Quds Medical Laboratories (Amman, Jordan). Commercial leptin enzyme immunoassay (EIA) kits were obtained for quantitative determination of serum leptin (DRG Diagnostics, Marburg, Germany) with analytical sensitivity of about 1.0 ng/mL, according to the manufacturer normal levels.
Hematology parameters
Clinical hematology parameters were measured for all volunteers, platelets count, total leukocyte count, differential leukocyte counts, hematocrit, hemoglobin and RBC indices (Mean Cell Hemoglobin [MCH], Mean Cell Volume [MCV], Mean Cell Hemoglobin Concentration [MCHC]), mean platelets count. Complete blood count was performed on the COBAS MICROS OT 18 (Roche, France).
Body mass index
On the evaluation day, height (cm) and weight (kg) of participants were recorded. Then, body mass index (BMI, kg/m2) was calculated accordingly.
Statistical analysis
Statistical analysis was performed using a statistical software package SPSS, version 19.0 for Windows (Chicago, IL, USA). Paired t-test was conducted to compare the differences of demographic and clinical findings between the means of the two study groups. Pearson analysis was used to find if there was any correlation between the participants’ characteristics and serum leptin levels.
Results
Study design and participants
One hundred and twenty-two healthy male pharmacy students with age between 18 and 24 years were invited to participate in this study. Samples were taken from only ninety four (94) participants who committed to follow the study protocol as presented in Figure 1. Four (4) samples were excluded due to insufficient quantity of saliva. Participants were categorized into two groups, a NL group (n=36, 45.4%) and a HN group (n=41, 54.6%).
Descriptive characteristics
As reported in Table 1, the comparison of the mean difference between the two groups was performed using t-test. In addition to serum leptin (p<0.0001), significant differences were noted between the NL and HN groups for the following factors (p<0.0001); M-DHEA, body weight, BMI, TG. T-test also showed significant difference between NL and HL groups in LDL (p<0.001) and TC (p =0.002). The mean age for HL participants was 22.1 (± 1.6) years, whereas it was 22 (± 1.73) years for NL participants.
| Variable | NT | HL | P-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (year) | 22 ± 1.73 | 22.1 ± 1.6 | 0.756 |
| Leptin | 4.1 ± 2.2 | 19.2 ± 8.7 | <0.0001 |
| sT (pmol/L) | 242.4 ± 158.3 | 287.2 ± 155.7 | 0.175 |
| M_CORT (ng/mL) | 8.7 ± 3.7 | 9.2 ± 2.9 | 0.393 |
| N_CORT (ng/mL) | 4.6 ± 2.1 | 5.4 ± 1.7 | 0.944 |
| M_DHEA (ng/mL) | 1.6 ± 0.5 | 1.2 ± 0.5 | <0.0001 |
| N_DHEA (ng/mL) | 1.033 ± 0.49 | 1.1 ± 0.4 | 0.188 |
| Body Weight (kg) | 71 ± 8.6 | 89.9 ± 13.7 | <0.0001 |
| Height (cm) | 175.1 ± 6.3 | 175.7 ± 5.9 | 0.602 |
| BMI (kg/m2) | 23.2 ± 2.2 | 29.1 ± 4.2 | <0.0001 |
| FBG (mg/dL) | 87.1 ± 8.3 | 86.4 ± 8.8 | 0.654 |
| TG (mg/dL) | 115.6 ± 42.8 | 157.7 ± 51.8 | <0.0001 |
| Total Cholesterol (mg/dL) | 167.8 ± 28.3 | 185 ± 28.5 | 0.002 |
| HDL-C (mg/dL) | 50.8 ± 7.8 | 48.7 ± 7.1 | 0.151 |
| LDL-C (mg/dL) | 96.5 ± 28.4 | 115.8 ± 23.1 | <0.001 |
| HGB | 15.8 ± 1.1 | 16.1 ± 1.4 | 0.157 |
| PCV | 46.4 ± 4.8 | 46.2 ± 4.8 | 0.808 |
| LYMPH | 34.7 ± 7.2 | 36.2 ± 6.5 | 0.282 |
Note. sT: salivary testosterone; M: Morning; N: Night; CORT: Cortisol; BMI: Body Mass Index; FBG: Fasting Blood Glucose; HDL-C: High-Density Lipoprotein Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol; TG: Triglycerides; MCH: Mean Cell Hemoglobin; MCHC: Mean Cell Hemoglobin Concentration; MCV: Mean Cell Volume; PCV: packed Cell Volume; RBC: Red Blood Cell; WBC: White Blood Cell
Table 1: Characteristics of the two study groups subdivided by their serum leptin levels (Mean ± Standard Deviation).
Correlations of salivary steroid hormones in all study subjects, NL and HL study groups: Correlation coefficients test was used to study the associations between all variables including SSH and serum leptin. As reported in Table 2, correlation coefficients model analysis found that M-DHEA was significantly positively correlated with serum leptin in all subjects regardless of study group (r=-0.248, p=0.003). The model analysis also found that body weight showed greater significant and positive correlation with serum leptin level than did BMI (NL: r=0.549 vs. 0.429, HL; r=0.517 vs. 0.422). Among lipid profile and regardless of the distribution of the two study groups, TG showed the closest correlation with serum leptin (r=0.367, p<0.0001), LDL (r=0. 0.318, p<0.001) and TC (r=0. 0.303, p<0.001). HDL-C level did not show any significant statistical relationship with serum leptin (r=-0.049, p=0.619).
| NL | HL | All subjects | ||||
|---|---|---|---|---|---|---|
| Variable | ||||||
| R | P-value | R | P-value | R | P-value | |
| Age | -0.068 | 0.606 | 0.144 | 0.334 | 0.069 | 0.477 |
| sT | 0.141 | 0.356 | -0.246 | 0.095 | 0.017 | 0.875 |
| M_COR | 0.032 | 0.813 | 0.133 | 0.374 | 0.116 | 0.238 |
| N_CORT | -0.032 | 0.818 | 0.217 | 0.060 | 0.090 | 0.375 |
| M_DHEA | -0.037 | 0.785 | 0.013 | 0.931 | -0.248** | 0.003 |
| N_DHEA | -0.240 | 0.084 | 0.255 | 0.083 | -0.142 | 0.158 |
| Weight | 0.429** | 0.001 | 0.549** | <0.0001 | 0.743** | <0.0001 |
| Height | 0.104 | 0.426 | -0.030 | 0.840 | 0.042 | 0.667 |
| BMI | 0.422** | 0.001 | 0.517** | <0.0001 | 0.750** | <0.000 |
| FBG | 0.054 | 0.679 | 0.149 | 0.319 | 0.033 | 0.738 |
| TG | 0.127 | 0.328 | 0.080 | 0.595 | 0.367** | <0.000 |
| Chol | 0.164 | 0.171 | 0.149 | 0.318 | 0.303** | 0.001 |
| HDL | 0.123 | 0.351 | 0.121 | 0.418 | -0.049 | 0.619 |
| LDL | 0.063 | 0.628 | 0.117 | 0.435 | 0.318** | 0.001 |
| HGB | 0.015 | 0.910 | 0.040 | 0.790 | 0.127 | 0.191 |
| PCV | 0.010 | 0.940 | -0.009 | 0.954 | -0.021 | 0.832 |
| LYMPH | 0.075 | 0.584 | -0.065 | 0.665 | 0.069 | 0.494 |
| Neutrophila | 0.036 | 0.796 | 0.050 | 0.739 | -0.038 | 0.707 |
| PLT | -0.006 | 0.965 | 0.066 | 0.658 | 0.060 | 0.552 |
** Correlation is significant at the 0.01 level (2-tailed)
* Correlation is significant at the 0.05 level (2-tailed)
Note. B: Slope; BMI: Body Mass Index; FBG: Fasting Blood Glucose; HDL-C: High-Density Lipoprotein Cholesterol; LCI:
Lower 95% Confidence Interval; UCI: Upper 95% Confidence Interval; LDL-C: Low-Density Lipoprotein Cholesterol; MCH:
Mean Cell Hemoglobin; MCHC: Mean Cell Hemoglobin Concentration; MCV: Mean Cell Volume; N: Sample Size; PCV:
Packed Cell Volume; R: Pearson Linear Correlation Coefficient; R2: Determinant Coefficient; RBC: Red Blood Cell; sT:
Salivary Testosterone, TG: Triglycerides; WBC: White Blood Cell
Table 2: The Pearson correlation between subjects’ characteristics and leptin serum level in NL, HL and all subjects irrespective of normality of serum level of leptin.
M-DHEA correlations with study variables parameters that showed significant correlations with serum leptin were tested using Pearson correlation. Among these parameters; body weight, BMI and TG showed negative and significant correlation with M-DHEA (r=- 0.26, p=008; r=-263, p=0.004; r=-0.283, p=0.004), respectively whereas TC and LDL levels were not significantly correlated M-DHEA (Table 3).
| Variable | Pearson correlation coefficient | p-value |
|---|---|---|
| Weight | -0.260** | 0.008 |
| BMI | -0.283** | 0.004 |
| TG | -0.283** | 0.004 |
| TC | -0.067 | 0.497 |
| LDL | -0.052 | 0.598 |
** Correlation is significant at the 0.01 level (2-tailed) * Correlation is significant at the 0.05 level (2-tailed)
Table 3: Pearson correlation between some subjects’ characteristics and their M_DHEA in all subjects of study groups (n=90).
Discussion
This study examined the relationship between serum leptin as an adiposity marker and salivary steroid hormones as a stress marker in a representative sample of Arab university male students. In line with our hypotheses and consistent to our predictions, salivary levels of M-DHEA showed a negative correlation with adiposity predictors such as serum leptin levels and BMI values. The steroid hormones differences observed among the students may reflect obesogenic differences in exposure to stress and coping with stress among university students. In the same context and except for M-DHEA, hyperleptinemic students with greater BMI had higher morning salivary steroid levels, compared with NL group. These changes in steroidal hormones levels can be attributed to student's maladaptation to stress. Prior studies have described that an antagonistic relationship between DHEA and cortisol is present as part of the long-acting body's stress hormones consequence [32,33].
DHEA is thought to be an anti-glucocorticoid hormone [15]. It antagonizes neurotoxic effects of cortisol on the hippocampus [34] or at least, in clinical trials, reduces circulating levels of cortisol [20,21]. Accordingly, DHEA has a potential stress-buffering role and this indicates a strong association between cortisol and DHEA which is fully functional in young people but deficient in old people [35,36].
It has been reported that chronic stress is accompanied with elevated cortisol and declined DHEA levels. However, DHEA/cortisol imbalance that reflects stress maladaptation remains a debatable point. Although, it was noted that DHEA levels decline primarily with the individual's age [37] noted that DHEA production in adults was normal. Notably, consistent with our findings, a recent study shown that a high cortisol to DHEAS ratio increases the risk of sickness absence in Japanese adult male workers over 50 years [18]. Moreover, a higher ratio of DHEAS to cortisol observed in the Tai Chi practitioners may predict term of improving an individual’s healthrelated quality of life during the aging [38]. Overall, it seems that DHEA/cortisol imbalance isn't only mediated by the influence of age as a single factor but may be also driven by youth associated factors such as adiposity. DHEA has anti-obesogenic effect where it can improve glucose tolerance by converting excess body fat to lean muscle mass [39]. This may explain the negative associations between diurnalnight levels of DHEA and leptin as has been shown [26-40]. These observations are consistent with [40] who have linked DHEA/cortisol imbalance to the obesity parameter in subjects with metabolic syndrome.
On the other hand, the lack of a positive association between DHEA and testosterone as anabolic hormones in our study is problematic to understand, without linking it to leptin. To a certain extent our results were similar to a recent study which reported that obesity but not aging was associated with a decrease in T over time in healthy men with an age of 40 and over [41]. This supports the effect of fat mass on men's testosterone levels. Furthermore, obesity was associated with the risk of T deficiency, but in men with BMI less than 30 kg/m2 had no change in levels of T over time [42].
Conclusion
The results of our study allow to determine the relationship between obesity and testosterone in the sample of university male students in order to compare it with other age groups. A positive correlation between DHEA and testosterone has been shown in recent study but the increase in T was more marked in women (p<0.001) than men (p<0.05) [43]. Also, regardless of serum leptin levels, a positive relationship between hyperleptinemia and hyperlipidemia was noted with significantly higher levels of serum TG levels in HL students compared to their NL.
Recently, but conversely to Gates et al.'s [44] findings, we observed that leptin was positively correlated with salivary testosterone in another stress-cohort study conducted on Arab young men with sleep deprivation [14]. Nevertheless, our findings draw some attention on the potential mechanisms linking obesity, stress and aging hormones in young men. Since leptin as the main obesity marker that promotes steroid hormones imbalance leading to maladaptation to chronic stress in young men. We observed that serum leptin levels are proportional to DHEA/cortisol imbalance and suggested to be a new indicator of chronic stress maladaptation in obese male young men.
Acknowledgment
Authors are grateful to the Applied Science Private University, Amman, Jordan for the full financial support granted to this research project (DRGS: 2007-7).
References
- Dallman MF, Pecoraro N, Akana SF, et al. (2003). Chronic stress and obesity: A new view of‘comfort food’. Proc Natl Acad Sci. 100: 11696-701.
- Cavagnini F, Croci M, Putignano P, et al. (2000). Glucocorticoids and neuroendocrine function. Int J Obes Relat Metab Disord. 2: 77-79.
- Adam TC, Epel ES. (2007). Stress, eating and the reward system. Physiol. Behav. 91: 449-458.
- Abu-Samak M, Khuzaie R, Abu-Hasheesh M, et al. (2008). Relationship of vitamin B12 Deficiency with overweight in male Jordanian youth. J Appl Sci. 8: 3060-3063.
- Marceau K, Zahn-Waxler C, Shirtcliff E A, et al. (2014). Within-adolescent coupled changes in cortisol with DHEA and testosterone in response to three stressors during adolescence. Psychoneuroendocrinology. 41: 33-45.
- Abu Khadra K, Khalil A, Abu-Samak M, et al. (2014) Evaluation of selected biochemical parameters in the saliva of young males using mobile phones. Electromagn Biol Med. 3: 72-6.
- Lee TK, Clarke IJ, St John J, et al. (2013). High cortisol responses identify propensity for obesity that is linked to thermogenesis in skeletal muscle. FASEB J. 28: 35-44.
- Park HK, Ahima RS. (2015). Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 64: 24-34.
- Abu-Hasheesh MO, Abu-Samak MS, Al-Matubsi HY, et al. (2010). Association of parental history of type 2 diabetes mellitus with leptin levels in Jordanian male youth. Saudi Med J. 31: 882-6.
- Leal-Cerro A, Considine RV, Peino R, et al. (1996). Serum immunoreactive-leptin levels are increased in patients with Cushing’s syndrome. Horm Metab Res. 28: 711-713.
- Krsek M, Silha JV, Jezková J, et al. (2004). Adipokine levels in Cushing’s syndrome; elevated resistin levels in female patients with Cushing’s syndrome. Clin Endocrinol (Oxf). 60: 350-357.
- Morton GJ, Schwartz MW. (2011). Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 91: 389-411.
- Abraham SB, Rubino D, Sinaii N, et al. (2013). Cortisol, obesity and the metabolic syndrome: a cross-sectional study of obese subjects and review of the literature. Obesity (Silver Spring). 21: 105-117.
- Abu-Samak MS, Mohammad BA, Abu-Taha MI, et al. (2018). Associations between sleep deprivation and salivary testosterone levels in male university students: A prospective cohort study. Am J Mens Health. 12: 411-419.
- Ebeling P, Koivisto VA. (1994) Review Physiological importance of dehydroepiandrosterone. Lancet. 11: 1479-1481.
- Fichna M, Fichna P, GryczyÃÆââ¬Â¦Ãâââ¬Å¾ska M, et al. (2015) Steroid replacement in primary adrenal failure does not appear to affect circulating adipokines. Endocrine. 48: 677-685.
- Korbonits M, Trainer PJ, Nelson ML, et al. (1996) Differential stimulation of cortisol and dehydroepiandrosterone levels by food in obese and normal subjects: relation to body fat distribution. Clin Endocrinol (Oxf). 45: 699-706.
- Hirokawa K, Fujii Y, Taniguchi T, et al . (2017). Association between cortisol to DHEA-s ratio and sickness absence in japanese male workers. Int J Behav Med.
- Alhaj HA, Massey AE, McAllister-Williams RH, et al (2006). Effects of DHEA administration on episodic memory, cortisol and mood in healthy young men: A double-blind, placebo-controlled study. Psychopharmacology (Berl). 188: 541-551.
- do Vale S, Martin Martins J, Fagundes MJ, et al. (2011). Plasma dehydroepiandrosterone-sulphate is related to personality and stress response. Neuro Endocrinol Lett. 32: 442-448.
- Maninger N, Wolkowitz OM, Reus, et al. (2009). Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol. 30: 65-91.
- Garfinkel SN, Ho SS, Wang X, et al. (2013). Cortisol facilitates memory by enhancing hippocampal activation and functional connectivity. Under review.
- Korbonits M, Trainer PJ, Nelson ML, et al. (1996). Differential stimulation of cortisol and dehydroepiandrosterone levels by food in obese and normal subjects: relation to body fat distribution. Clin Endocrinol (Oxf). 45: 699-706.
- Pinto A, Malacrida B, Oieni J, A, et al. (2015) DHEA modulates the effect of cortisol on RACK1 expression via interference with the splicing of the glucocorticoid receptor. Br J Pharmacol. 172: 2918-2927.
- Lai HM, Liu MSY, Lin TJ, et al. (2017) Higher DHEAS Levels Associated with Long-Term Practicing of Tai Chi. Chin J Physiol. 60: 124-130.
- Abu-Samak MS, Abu-Zaiton, Al-Jaberi A, et al. (2014). Morning salivary cortisol associates with elevated serum leptin levels in Jordanian young men with olive pollen induced allergic rhinitis. Br J Med Med Res. 4: 797-806.
- Maggio M, Cattabiani C, Lauretani F, et al. (2010). The concept of multiple hormonal dysregulation. Acta Biomed. 81: 19-29.
- Wittert G. (2014). The relationship between sleep disorders and testosterone in men. Asian J Androl. 16: 262-265.
- Seidell JC, Bjorntorp P, Sjostrom L, et al. (1990). Visceral fat accumulation in men is positively associated with insulin, glucose and C-peptide levels, but negatively with testosterone levels. Metabolism. 39: 897-901.
- Abu-Samak M, Yousef A, Al-Jarie A, et al. (2011). Lipid and hematological parameters in hyperleptinemic healthy Arab male youth in Jordan. Pak J Biol Sci. 14: 344-350.
- Friedewald WT, Levy RI, Fredrickson DS (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 18: 499-502.
- Allott KA, Yuen HP, Bartholomeusz CF, et al. (2017). Stress hormones and verbal memory in young people over the first 12 weeks of treatment for psychosis. Psychiatry Res. 21: 60-66.
- Pinto A, Malacrida B, Oieni J, et al. (2015). DHEA modulates the effect of cortisol on RACK1 expression via interference with the splicing of the glucocorticoid receptor. Br J Pharmacol. 172: 2918-2927.
- Cardounel A, Regelson W, Kalimi M. (1999). Dehydroepiandrosterone protects hippocampal neurons against neurotoxin-induced cell death: Mechanism of action. Proc Soc Exp Biol Med. 222: 145-149.
- Corsini E, Pinto A, Galbiati V, et al. (2014). Corticosteroids modulate the expression of the PKC-anchoring protein RACK-1 and cytokine release in THP-1 cells. PharmacolRes. 81: 10-6.
- Kroboth P, Salek F, Pittenger A, et al. (1999). DHEA and DHEA-S: A review. J Clin Pharmacol. 39: 327–348.
- Tannenbaum, C, Barrett-Connor E, Laughlin GA, et al. (2004). A longitudinal study of dehydroepiandrosterone sulphate (DHEAS) change in older men and women: The Rancho Bernardo Study. Eur J Endocrinol. 151: 717-25.
- Lai HM, Liu MSY, Lin TJ, et al. (2017). Higher DHEAS levels associated with long-term practicing of tai chi. Chin J Physiol. 30: 124-130.
- Korbonits M, Trainer PJ, Nelson ML, et al. (1996). Differential stimulation of cortisol and dehydroepiandrosterone levels by food in obese and normal subjects: Relation to body fat distribution. Clin Endocrinol (Oxf). 45: 699-706.
- Phillips AC, Carroll D, Gale CR, et al. (2010). Cortisol, DHEAS, their ratio and the metabolic syndrome: Evidence from the Vietnam experience study. Eur J Endocrinol. 162: 919-23.
- Sartorius GA, Nieschlag E. (2010). Paternal age and reproduction. Hum Reprod Update. 16: 65-79.
- Haring R, Ittermann T, Volzke H, et al. (2010). Prevalence, incidence and risk factors of testosterone deficiency in a population-based cohort of men: Results from the study of health in Pomerania. Aging Male. 13: 247-257.
- Collomp K, Buisson C, Gravisse N, et al. (2018). Effects of short-term DHEA intake on hormonal responses in young recreationally trained athletes: modulation by gender. Endocrine. 59: 538-546.
- Gates MA, Mekary RA, Chiu GR, et al. (2013). Sex steroid hormone levels and body composition in men. J Clin Endocrinol Metab.98: 2442-2450.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences