Reactive Oxygen Species in Inflammationn
Jes Paul
Jes Paul*
Department of Molecular and Cellular Physiology, Albany Medical College, Albany, NY, USA.
Received date: January 30, 2018; Accepted date: February 12, 2018; Published date: February 20, 2018
Citation: Paul J. Reactive Oxygen Species in Inflammation. Electronic J Biol, 14:1
Abstract
One of the key signaling molecules that play a major role in inflammation is ROS. Polymorphonuclear neutrophils enhance and activate the generation of ROS at the site of the endothelial dysfunction and tissue injury. The migration of inflammatory cells from blood to tissue is facilitated by the vascular endothelium. Followed by the inflammation there is opening of indo-endothelial junction through which migration of inflammatory cells takes place. The migration of inflammatory cells across the endothelial barrier not only clears the way to pathogens and foreign particles but also causes serious tissue injury. This review highlights the oxidative stress mediated signaling mechanisms involved in inflammation leading to serious illnesses like diabetes.
Keywords
Inflammation; Neutrophil; Immune System; Cytokines.
Introduction
Inflammation is highlighted as a defective immune response that is conferred by the host against the foreign pathogens. The body’s own inbuilt immune system response which is triggered under the encountering of the pathogenesis is referred as innate immunity. This elicits many acute inflammatory responses accompanied by systemic vasodilation, vascular leakage and leukocyte immigration [1]. according to the roman physician Celsius, the four cardinal signs of localized acute inflammation are calor, heat, rubor, redness, tumor swelling and dolor pain leading to impairment of function.
The recognizing of the wide range of pathogens by the innate immune system is due to the presence of germ-line encoded receptor known as Pattern- Recognition Receptors (PPRS) [2]. TLRS (Toll like Receptors) C type lectin receptors and NLR (Cytoplasmic Nod like Receptors) came under the category of PPRS. Those receptors recognize the pathogen associated molecular patters as well as the danger associated molecular patterns released by the mechanism of dsDNA and uric acid crystals. The PRRS are expressed as a variety of immune cells includes macrophages, neutrophils, monocytes and DCs (dendritic cells) helping in the early detection of the pathogens [2].
Followed by the activation of the immune response, the activation of acute immune response takes place resulting in the secretion of cytokines and chemokines. The first cells that act is neutrophils, which by adhering to the endothelial wall and later migrating to vascular wall at the site of infection, engulf the invading pathogens. It also secrets vasoactive as well as pro-inflammatory mediators [3]. The early vascular change at the site of infection is due to the pro inflammatory mediators [1]. The mediators include histamine, PAFS (platelet activating factor, bradykinins and thrombins. These increase the vascular permeability followed by fluid accumulation (edema) and leukocyte extravasation. If innate immune system exceeds its capacity or if its defensive capacity is limited, the adaptive immune system is engaged acting specific T and B cells for pathogen clearance [1]. If this process also gets inefficient it progress to chronic state of inflammation. This is associated with many diseases such as diabetes and many heart diseases.
The center of progression of many inflammatory disease is the production of ROS. The PMNS (polymorphonuclear neutrophils) are the ones which produce ROS. This promotes the oxidation of cellular signaling proteins such as tyrosine phosphatases promoting endothelial dysfunction. The two roles played by ROS in inflammation are as a signaling molecule and a mediator. Superoxides like ROS can easily diffuse with NO and can form RNS (reactive nitrogen species). This induces nitrosative stress which adds to the proinflammatory burden of ROS. The focus of this review is the ROS dependent mechanism of inflammation leading to diseases like diabetes.
Ros and Its Regulation in Inflammation
These reduced metabolites of oxygen possessing strong oxidizing capabilities are injurious because they oxidize protein and lipid cellular constituents and damage the DNA. At physiological concentration they can function as signaling molecules for cell growth, adhesion of cell towards other cells, differentiation, senescence and apoptosis [4,5]. The central reason to the progression of inflammatory diseases is the prolonged ROS production [6].
The widely studies ROS are superoxide anion (O2-) Hydroxyl radical (OH), hydrogen peroxide (H2O2) and hypochlorous acid (HOCl). ROS are mainly generated as the by-products of cellular metabolism through electron transport chain (ETC) in mitochondria as well as via cytochrome P450. Another major source of ROS is not as the by-products but is the NADPH oxidases that are present in a variety of cells, especially the professional phagocytes and endothelial cells [7], which are central to the genesis of inflammatory response. Enzymatic catalysis of NADPH oxidase, xanthane oxidase (XO) or during electron transfer reactions which takes place in mitochondria ,uncoupled NOs derived reaction result in the generation of O2- [5,8,9].
In various experiment models, the role of oxidants in inducing inflammation is investigated. Even though the fundamental role of oxidants is known but how they contribute to response is still under study. Whether antioxidant therapy is valid in arresting inflammation in patients is studied widely, but it is still unresolved.
Induced cytokines (e.g. TNF α), the stress of hypoxia, ischemia-reperfusion injury, bacterial toxins (LPS) and mediators that litigate cell surface receptors (PAF, thrombin, histamine, VEGF and bradykinins) are the commonly used mediators to simulate inflammation.
Oxydative Stress and Tissue Injury
PHA is a family of bio-polyesters featuring diverse structures and it is the only bio-plastic that can be synthesized by microbes. PHA can be synthesized by more than 30% of the soil-borne bacteria [10]. These polymers accumulate intracellular for about 90% of the cell’s dry weight under nutritional stress conditions and they are served as the carbon resources and energy storage [1]. Some bacteria are active in the sludge in the sea floor regions and in extreme environments and they also have the ability to make PHA. During the past decade, PHA has been developed to serve various uses [11]. PHA’s molecular mass is about 50000 to 1000000 Dalton which is different from the same of the PHA producers. Monomeric units, as well, exist in D configuration as a result of the spatial features belonging to biosynthetic enzymes [12-15]. PHA has structure-related rich features. PHA’s homopolymers, random copolymers and blocking copolymers can be produced depending on the type of the bacteria and the growth conditions. More than 150 monomers have been reported for PHAs. PHAs have changeable mechanical and thermal features as well as use diversities such as in environment-favorable biodegradable plastics which are currently used for packing, fibers, degradable and biocompatible implants. They also facilitate drug releasing transporters and PHA monomers can also be used for the development of bio-fuels, drugs or chiral intermediates. PHA oligomers have also been reported as nutrients for animals. Due to these advances, microbial PHA composes a valuable chain of industrial fermentation, materials, drugs and biofuels to the excellent chemical compounds. More than 20 companies have been established worldwide to commercialize such progresses [16,17].
A characteristic feature of chronic inflammatory disease is the accumulation of activated macrophages at the site of injury [10]. Even though macrophages are meant to eliminate pathogens; if they are excessive and unchecked they can also lead to tissue injury. Based on the current classification there are two macrophages M1 (clinically activated macrophage) and M2 (alternatively activated macrophages). M1 contribute to tissue injury by releasing large quantities of highly reactive cytotoxic oxidants to destroy pathogens while M2 suppress inflammation and help in wound resolution by phagocytizing dead neutrophils and synthesizing molecules such as TGF-β, VEGF and EGF that are needed for tissue modeling [10,11].
M1 macrophages by producing high oxidative stress secrete pro inflammatory cytokines such as TNF α, IL1 and IL6 while M2 macrophage produce antiinflammatory cytokines IL4, IL10 and IL13 [10]. M1 mainly induce tissue injury by releasing chemokines and reactive oxidants which can induce cell death receptors, culminating in Caspase activation via either extrinsic pathway (i.e., mitochondria independent) or an intrinsic pathway (i.e., mitochondria dependent) [12]. Antioxidant GSH equilibrium is altered by the oxidative stress produced by macrophages64. The main part of the pathways of cell death is the activation of Caspases.
Extrinsic Pathway
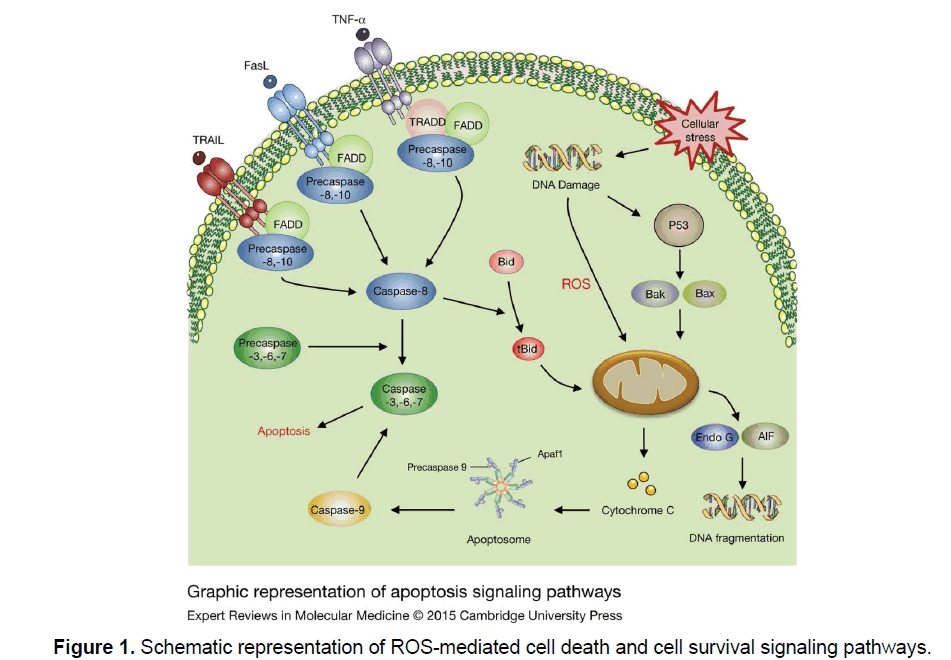
This pathway is mediated by cell death receptors. Those receptors bind with their ligands initiate protein-protein interactions, resulting in activation of initiator Caspases. The four cell death receptors included are TNF receptor 1 (TNFR1), TNF-related apoptosis inducing ligand receptor 1 (TRAIL R1), TRAIL receptor 2 (TRAIL R2) and FAS receptor. The ligands that bind to the receptors belong to TNF super family of cytokines are TNF-α, FAS ligand and TRAIL [12]. Excessive ROS generation sustained JNK activation and Caspase activation takes place with TNF-α induced cell death (Figure 1) [13-15].
TNF-α, a principal pleiotropic cytokine secreted by activated macrophages has many biological properties such as production of inflammatory cytokines, cell proliferation and cell death [16]. TNFR1 signaling under resting condition is inhibited by 60-KD cytoplasmic protein known as silencer of death domain (SODD). TNF-α promotes activation of TNFR1, following this within minutes TNFR1- associated death domain (TRADD) and FAS associated death domain(FADD) are recruited for the collective death inducing signaling complex (DISC) is internalized. Endosomal disc is released to cytosol further recruits initiator Precaspase-8 resulting in activation [12]. The apoptosis induced by FasR enhance ROS-generation via NADPH oxidase and caspase activation similar to TNFR1.Whereas the key feature of FasR mediated apoptosis is regulated by FLICE inhibitory protein (FLIP), which inhibits apoptosis by binding of Procaspase-8 to FADD [17]. The enhanced ROS generation down regulates FLIP protein and executes FasR dependent apoptosis [18].
The essential determinant of whether cell undergoing mitochondrial dependent or mitochondria independent apoptosis depends on the extensive caspase 8 [19]. Extensive caspase activation directly activated effective Caspases such as Caspase3 and induced cell death, whereas low caspase-8 activation activated an amplification loop that was dependent on the mitochondria. However TNF-α doesn’t induce apoptosis in all cells because it can alternatively activate at least two cell survival pathways (Figure 1).
Intrinsic Pathway of Cell Death
These pathways depend on increased mitochondrial outer membrane permeability (MOMP) which causes mitochondria to cytosol release of apoptogenic proteins such as Cytochrome C (Cyt C), Smac/ Diabolo apoptosis inducing factor (AIF) and endonuclease G which trigger cell death in either a caspase dependent/caspase independent manner (Figure 1) [20,21]. This phenomenon of increased MOMP can be induced by excessive Ca2+ entry ,high oxidative stress and compounds which result in mitochondrial membrane depolarization [22]. The released Cyt C then binds to apoptosis activation factor (APAF 1) and recruits initiator Pro-caspase 9 which undergoes auto-activation. This whole complex collectively known as “Apoptosome” (Figure 1) [20]. The effective Caspases such as Caspase 3 is activated by Caspase 9.Smac/Diabolo also facilitates the activation of effector Caspases by removing the blockage by Inhibitor of apoptosis proteins (APS) [22].
DNA condensation and cleavage is induced by AIF activation [23]. EndoG-mediated DNA fragmentation is the final event in the apoptosis. The mitochondrial release of Cyt C is considered the major determinant of cell death.
Conclusion
Intricate relationship between oxidative stress and inflammation has been described by various intensive researches in to mechanisms of inflammation. The principle objective of inflammation is the clearance of pathogen from the body. In this process the fundamental role is played by the ROS produced by the phagocytic cells. For maintaining homeostasis ambient level of ROS is needed whereas for killing pathogens excessive ROS is needed, uncontrolled generation of ROS leads to tissue injury [24]. So the generation of ROS plays a crucial role in inflammation. It is also important to the pathogenesis of tissue injury. Even though the role of ROS in chronic inflammatory diseases such as diabetes, heart diseases is known, the how they contribute to the mechanism is still under study.
References
- Clark R, Kupper T. (2005). Old meets new: The interaction between innate and adaptive immunity. J Invest Dermatol. 125: 629-637.
- Takeuchi O, Akira S. (2010). Pattern recognition receptors and inflammation. Cell. 140: 805-820.
- Kolaczkowska E, Kubes P. (2013). Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 13: 159-175.
- Tichá T, Lochman J, ÃÆââ¬Å¾Ãâà âinÃÆââ¬Å¾ÃâÃÂalová L, et al. (2017). Redox regulation of plant S-nitrosoglutathione reductase activity through post-translational modifications of cysteine residues. Biochem Biophys Res Commun. 494: 27-33.
- Thannickal VJ, Fanburg BL. (2000). Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 279: 1005-1028.
- Griffith B, Pendyala S, Hecker L, et al. (2009) NOX enzymes and pulmonary disease. Antioxid Redox Signal. 11: 2505-2516.
- Pendyala S, Natarajan V. (2010). Redox regulation of Nox proteins. Respir Physiol Neurobiol. 174: 265-271.
- Handy DE, Loscalzo J. (2012). Redox regulation of mitochondrial function. Antioxid Redox Signal. 16: 1323-1367.
- Müllebner A, Dorighello GG, Kozlov AV, et al. (2018). Interaction between mitochondrial reactive oxygen species, heme oxygenase and nitric oxide synthase stimulates phagocytosis in macrophages. Front Med (Lausanne). 22: 252.
- Laskin DL, Sunil VR, Gardner CR, et al. (2011). Macrophages and tissue injury: Agents of defense or destruction? Annu Rev Pharmacol Toxicol. 51: 267-288.
- Lee S, Zhang J. (2012). Heterogeneity of macrophages in injured trigeminal nerves: Cytokine/chemokine expressing vs. phagocytic macrophages. Brain Behav Immun. 26: 891-903.
- Circu ML, Aw TY. (2010). Reactive oxygen species, cellular redox systems and apoptosis. Free Radic Biol Med. 48: 749-762.
- Yan H, Xiao F, Zou J, et al. (2018). NR4A1-induced increase in the sensitivity of a human gastric cancer line to TNFα-mediated apoptosis is associated with the inhibition of JNK/Parkin-dependent mitophagy. Int J Oncol. 52: 367-378.
- Sakon S, Xue X, Takekawa M, et al. (2003). NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 22: 3898-3909.
- Woo CH, Eom YW, Yoo MH, et al. (2000). Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J Biol Chem. 275: 32357-32362.
- Karbach J, Neumann A, Brand K, et al. (2012). Phase I clinical trial of mixed bacterial vaccine (Coley's toxins) in patients with NY-ESO-1 expressing cancers: Immunological effects and clinical activity. Clin Cancer Res. 18: 5449-5459.
- He MX, He YW. (2015). c-FLIP protects T-lymphocytes from apoptosis in the intrinsic pathway. J Immunol. 194: 3444-3451.
- Wang L, Azad N, Kongkaneramit L, et al. (2008). The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J Immunol. 180: 3072-3080.
- Kutumova E, Zinovyev A, Sharipov R, et al. (2013). Model composition through model reduction: A combined model of CD95 and NF-κB signaling pathways. Syst Biol. 7: 13.
- Green DR, Kroemer G. (2004). The pathophysiology of mitochondrial cell death. Science. 305: 626-629.
- Kroemer G, Reed JC. (2000). Mitochondrial control of cell death. Nat Med. 6: 513-519.
- Tsujimoto Y, Shimizu S. (2007). Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 12: 835-840.
- Delavallée L, Cabon L, Galán-Malo P, et al. (2011). AIF-mediated caspase-independent necroptosis: A new chance for targeted therapeutics. IUBMB Life. 63: 221-232.
- Bartesaghi S, Radi R. (2018). Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 14: 618-625.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences