Phytochemical Screening and Antioxidant Activity of Edible Wild Fruits in Benguet, Cordillera Administrative Region, Philippines
Racquel Barcelo
Faculty,Department of Biology,School of Natural Sciences,Saint Louis University,Bonifacio St.,Baguio City,Philippines.
Abstract
This study identified the secondary metabolites present and determined the antioxidant activity of 31 edible wild fruits grown in Benguet province, Cordillera Administrative Region, Philippines. Total polyphenol and flavonoid content were estimated using Folin-Ciocalteu and aluminium chloride method respectively. Antioxidant activity was measured through diphenyl-1-picrylhydrazyl assay. Based on the results, the following bioactive constituents are present in the fruits: alkaloids, steroid glycosides, saponins, flavonoids, polyphenols and tannins. The fruits contain more polyphenols than flavonoids. All the fruits except Physalis peruviana (Solanaceae) exhibited higher antioxidant activity than Vitamin E (Myra E), ascorbic acid (50 ug/mL), and trolox (1000 uM). Dillenia philippinensis (Dilleniaceae) exhibited the highest antioxidant activity. The antioxidant activity of the fruits and controls is significantly different (ρ ≤ 0.05). Post-hoc Tukey analysis of data reveals that several fruits have equal activity. Finally, there is a positive moderate correlation (r=0.50) between the total polyphenol content and antioxidant activity of the fruits.
Keywords
Diphenyl-1-picrylhydrazyl (DPPH) activity; Edible wild fruits; Natural antioxidants; Secondary metabolites.
Introduction
Globally,there’s a continuous trend in identifying the most beneficial dietary fruits. The Philippines,as a tropical country,boasts of rich and diverse plant resources. In the Cordillera region,Benguet province is richly endowed with a wide variety of wild fruits. These fruits are edible but often neglected and underutilized.
The interest on the secondary metabolites present in wild fruits is increasing [1]. Numerous studies have revealed the antioxidant activities of phytochemicals found in wild fruits that suggest their positive role in the prevention of diseases [2-4]. Antioxidants are chemical substances that inhibit oxidation process by preventing the formation of free radicals that cause damage to healthy cells [5]. Fruit consumption reduces risks of chronic degenerative diseases such cancer [6]. In the Philippines,cancer is the third leading cause of death with mortality rates of up to 50,000 deaths among Filipinos and growing by five percent every year [7]. It is the leading cause of death worldwide projecting an estimated number of 12.1 M in 2030 [8]. Up to this date,there is no information on the secondary metabolites,total phenolic content and antioxidant activity of edible wild fruits in Benguet province. As a result,this study was carried out.
Methods
Collection,transport and storage of fruits
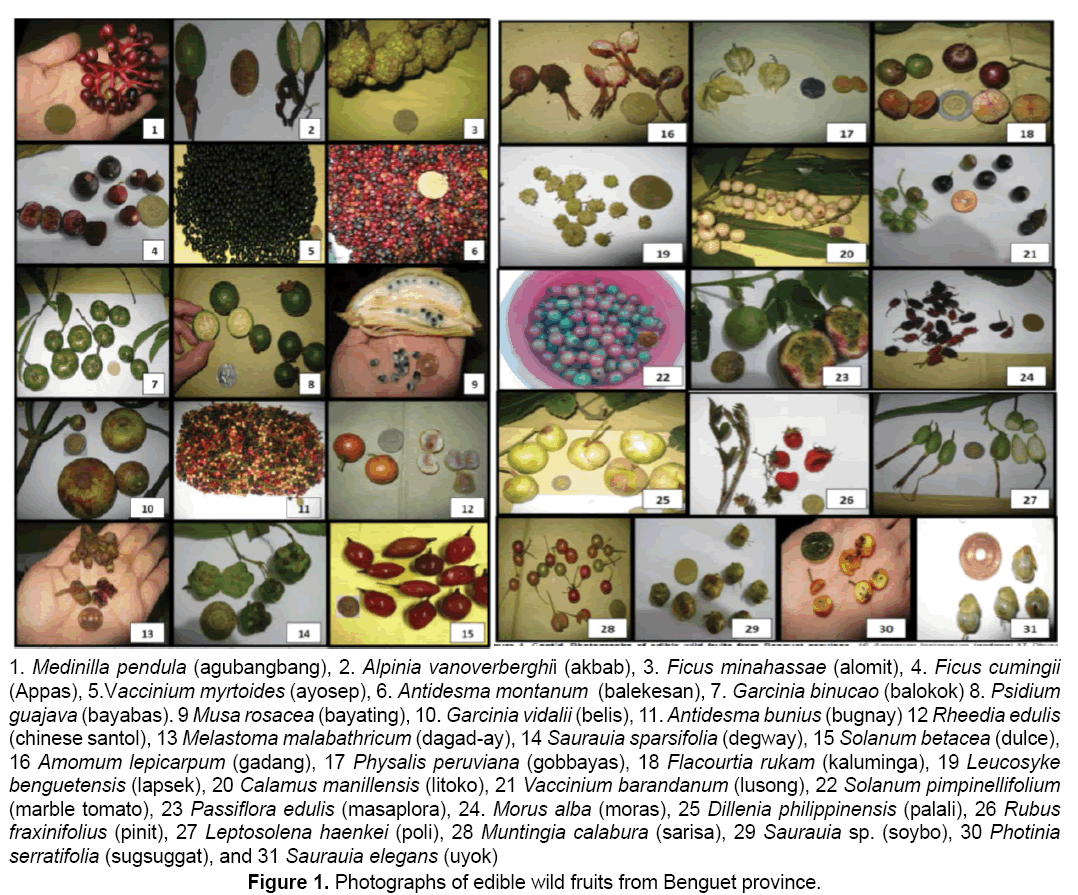
Fresh ripe,edible wild fruits (1 kg) were randomly collected by hand picking with the help of some field assistants from selected barangays of the different municipalities of Benguet with gratuitous permit no. DENR-CAR 005-13. Random sampling was carried out which involved taking any ripe fruit to collect in sufficient quantity [9,10]. Through this method,a diverse range of ripe fruit was sampled and collection of a large number of fruits was done quickly [11]. The fruits were packed using zip lock plastic bags and stored into an ice box which is cool,dark and moist. Fresh fruits were delivered to the Natural Sciences Research Unit laboratory in Saint Louis University,Baguio City. In the laboratory,the fruits were kept in an ultra low freezer (Legaci,USA) at -20°C until analysis [12,13]. A total of 31 fruits were collected (Figure 1). Samples were botanically authenticated by Dr. Teodora Balangcod,botanist from the Northern Luzon University Herbarium at the University of the Philippines,Baguio. Voucher specimens (SLUHwere deposited to the Fr. Gerard Braeckman Museum of Natural History in Saint Louis University,Baguio City.
Preparation of fruit extracts
Fruits were removed from the freezer and allowed to thaw overnight at 20°C before analysis was performed [14]. The fresh fruits were washed initially using running tap water followed by distilled water. Only the edible portion of each fruit including seeds and peelings (20 g) was used for the preparation of extract. Parts were homogenized in 50 mL (80% v/v) methanol (Merck,Germany) using a blender (Kyowa 1000) for 5 minutes and soaked for 48 hours. Extract was filtered using Whatman No. 2 filter paper and filtrate was centrifuged using an automated centrifuge (Heraeus) at 5300 rpm for 10 minutes. Supernatant was stored at 4°C prior to use within 2 days [4].
Phytochemical screening
The secondary metabolites such as alkaloids,steroids,anthraquinones,saponins,polyphenols,flavonoids,and tannins were determined in each fruit sample using preliminary and confirmatory tests by Aguinaldo et al. [15]. The presence of alkaloids in the fruit samples was detected using Mayer’s and Dragendorff’s tests. Steroids using Keller-Killiani test for deoxysugars,Liebermann-Burchard test for unsaturated sterols and Kedde test for unsaturated lactones. Anthraquinones using Borntrager’s and Modified Borntrager’s tests. Flavonoids such as leucoanthocyanins using Bate Smith and Metcalf test. Flavonoids containing cyanidin-?-benzopyrene nucleus using Wilstatter “cyanidin” test. Froth test for saponins and Liebermann-Burchard test for unsaturated sterols and triterpenes. Further,tannins and polyphenols using Gelatin and Ferric chloride tests.
Determination of total polyphenols using folin ciocalteu assay
In 250 μL of methanolic extract,2,250 μL distilled water and 250 μL Folin-Ciocalteu reagent (Merck,Germany) were added and allowed to stand for reaction up to 5 minutes. This mixture was neutralized by 2,500 μL of 7% sodium carbonate (w/v) (HiMedia,India) and was kept in the dark at room temperature for 90 minutes. The absorbance of resulting blue color was measured at 765 nm using VIS spectrophotometer (Labomed Inc,USA). Quantification was done on the basis of standard curve of gallic acid (Merck,Germany) prepared in 80% methanol (v/v) (Merck,Germany) and results were expressed in milligrams GAE per 100 grams fw of fruits [4,16]. The standard curve was prepared using 0,50,100,150,200,250 mg L-1 solutions of gallic acid in methanol: water (50:50 v/v) [17,18].
Determination of total flavonoids using aluminum chloride method
Briefly,500 μL of methanolic extract of sample was diluted with 1,500 μL of distilled water and 500 μL of 10% w/v aluminum chloride (Ajax,Australia) added along with 100 μL of 1M potassium acetate (Calbiochem,San Diego CA) and 2,800 μL of distilled water. This mixture was incubated at room temperature for 30 minutes. The absorbance of resulting reaction mixture was measured at 415 nm VIS spectrophotometer (Labomed Inc.,USA). A yellow color indicated the presence of flavonoids [16]. Quantification of flavonoids was done on the basis of standard curve of quercetin (Calbiochem,San Diego CA) prepared in 80% methanol (Merck,Germany) and results were expressed in milligram QE per 100 grams fw of fruits [4,16]. The calibration curve was plotted by preparing the quercetin solutions at concentrations 25,50,75,100 and 125 ug/ml using 10 mg of quercetin dissolved in 80% methanol (Merck,Germany) [19].
Screening of antioxidant activity using 2,2-diphenyl-1-picrylhydrazyl assay
A solution of 0.2 mM DPPH (Sigma Aldrich,USA) in 80% methanol (Merck,Germany) was prepared in aluminum foil- wrapped test tube and 3 mL of this solution was mixed with 100 μL of extract in methanol. The reaction mixture was shaken thoroughly for 1 minute using a vortex mixer (VM 1000,Digisystem Lab Instruments Inc.) and left in the dark at room temperature for 30 minutes. The absorbance of the mixture was measured using a VIS spectrophotometer (Labomed Inc.,USA) at 517 nm. The ability to scavenge DPPH radical was calculated by the following equation: DPPH radical scavenging activity (%)= {[(Absneg control – Abssample)]/ (Absneg control )}x 100 where Absneg control is the absorbance of DPPH radical and methanol; Abssample is the absorbance of DPPH radical + fruit extract/ control [20-22].
Statistical analysis
The qualitative data obtained from phytochemical screening were interpreted and analyzed by comparing the secondary metabolites present in each fruit. The quantitative data on the total polyphenol content in mg GAE/ 100 grams fw and total flavonoid content in mg QE/ 100 grams fw of the fruits measured using Folin Ciocalteu assay and aluminum chloride method respectively were used to rank the fruits. Total polyphenol and flavonoid content of the fruits were calculated through linear regression. One way analysis of variance (ANOVA) was used at 0.05 level of significance to determine if there is a significant difference in the antioxidant activity between and among the fruits and controls using DPPH assay as indicated by % DPPH radical scavenging activity. Tukey test was performed using SPSS 18.0 for Windows software package. This post hoc test was used to identify where the significant difference lies between and among the fruits and controls. The relationship between the total polyphenol and flavonoid content of the fruits to their antioxidant activity using DPPH assay was analyzed using CORREL statistical function in MS Excel software as indicated by the Pearson correlation coefficient (r). The experimental results for all assays done were expressed as mean of three replicates.
Results and Discussion
Phytochemical constituents of fruits
All the fruits eaten by the local people in Benguet contain secondary metabolites (Table 1).
| Family | Fruit Source | A | S | N | F | P | T | O |
|---|---|---|---|---|---|---|---|---|
| Actinidiaceae | Saurauia sp. (soybo) | - | + | - | + | + | + | + |
| Saurauiaelegans | - | + | - | + | + | + | + | |
| Saurauiasparsifolia (degway/sapuwan) | - | + | - | + | + | + | + | |
| Arecaceae | Calamusmanillensis (litoko) | - | + | - | + | + | + | + |
| Clusiaceace | GarciniaBinucao (balokok) | - | + | - | + | - | + | - |
| Rheediaedulis (Chinese santol) | - | + | - | + | + | + | + | |
| Garciniavidalii (belis) | - | + | - | + | - | + | + | |
| Dilleniaceae | Dilleniaphilippinensis (palali) | + | + | - | + | - | + | + |
| Ericaceae | Vacciniumbarandanum (lusong) | - | + | - | + | + | + | - |
| Vacciniummyrtoides (ayosep/gotmo) | + | + | - | + | - | + | + | |
| Flacourtiaceae | Flacourtiarukam | - | + | - | + | + | + | - |
| Melastomataceae | Medinillapendula (agubangbang) | + | + | - | + | + | - | + |
| Melastomamalabathricum (dagad-ay) | - | + | - | + | + | + | + | |
| Moraceae | Appas (Ficuscumingii) | + | + | - | + | + | - | + |
| Ficusminahassae (alomit) | - | + | - | + | + | + | + | |
| Morus alba (moras) | - | + | - | + | + | + | - | |
| Muntingiaceae | Muntingiacalabura (sarisa) | - | + | - | + | + | + | - |
| Musaceae | Musa rosacea (bayating/amoting) | - | + | - | + | + | + | - |
| Myrtaceae | Psidiumedulis (wild guava) | - | + | - | + | + | + | + |
| Passifloraceae | Passifloraedulis (masaplora) | + | + | - | + | + | + | - |
| Phyllanthaceae | Antidesmabunius (bugnay) | - | + | - | + | - | + | - |
| Antidesmamontanum(balekesan) | - | + | - | + | + | + | + | |
| Rosaceae | Photiniaserratifolia (sugsuggat) | - | + | - | + | + | + | |
| Rubusfraxinifolius (pinit/doting) | - | + | - | + | + | + | ||
| Solanaceae | Solanumbetacea (dulce/tamarillo) | + | + | - | + | + | - | + |
| Physalisperuviana (gobbayas) | + | + | - | - | + | - | + | |
| Solanumpimpinellifolium (marble tomato) | + | + | - | + | + | - | + | |
| Urticaceae | Leucosykebengueensis (lapsek) | + | + | - | + | + | + | + |
| Zingiberaceae | Alpiniavanoverberghii (akbab) | - | + | - | + | + | + | + |
| Amommumlepicarpim (gaddang) | - | + | - | + | + | + | + | |
| Leptosolenahaenkei (poli) | - | + | - | + | + | + | - |
A-Alkaloids; S-Steriods; N-Anthraquinones; P- Saponins; F-Flavonoids; OPolyphenols; and T-Tannins (+ presence, - absence)
Table 1: Phytochemical constituents of the edible wild fruits.
Specifically,alkaloids are present in D. philippinensis,V. myrtoides,M. pendula,F. cumingii,P. edulis,S. betacea,P. peruviana,S. pimpinellifolium and L. benguetensis. Alkaloids occur in fruits [23]. Fruitoccuring tetrahydro-betacarboline alkaloids acted as antioxidants and free radical scavengers in the 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) assay when compared with ascorbic acid and trolox [24]. Steroids are present in all the fruits. Steroid glycosides isolated from berries of Solanum aculeastrum possess antioxidant activities using DPPH,ABTS and reducing power assays.
These glycosides are identified as tomatidine and solasodine [25]. Saponins were detected in all the fruits except G. binucao,G. vidalii,D. philippinensis,V. myrtoides and A. bunius. Saponins are widely distributed in plants [26]. Saponins from Solanum anguivi fruits exhibited antioxidant potential in Wistar rats [27]. Phenolics are present in all the fruits. Phenolics refer to flavonoids,tannins and polyphenols. These are found in all higher plants,often at high levels. These are commonly present in fruits,vegetables,wine and tea [28]. Tannins from grape and apple fruit have the ability to scavenge free radicals. The highest antioxidant activity was observed in the peels of Sampion cultivar of apples and white grapes [29]. In another study,tannins from Canarium album demonstrated potent antioxidant activity [30]. Flavonoids and polyphenols possess antioxidant properties as proven in numerous studies [31-35]. G.binucao (Clusiaceae) fruits are rich in steroids,flavonoids and tannins (polyphenols). Among all the fruits,L. benguetensis (Urticaceae) is the richest source of secondary metabolites because it contains all the secondary metabolites tested except anthraquinones.
Total polyphenol and flavonoid content of fruits
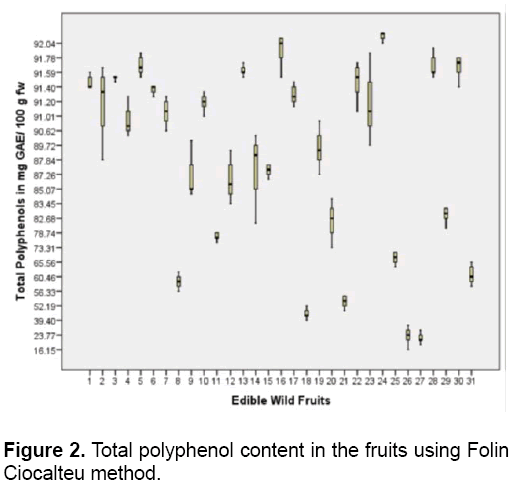
All the fruits contain polyphenols and flavonoids (Figures 2 and 3). Polyphenols are the most numerous group of secondary metabolites [36]. Phenolic compounds are subdivided into several classes such as flavonoids,tannins,phenolic acids (hydroxybenzoic and hydroxycinnamic acids) among others. These compounds are mainly derived from fruits aside from vegetables,cereals,legumes and nuts [37].In this study,R. fraxinifolius (Rosaceae) has the highest polyphenol content of 92.21 mg GAE/ 100 g fw followed by M. alba (Moraceae) ,L. benguetensis (Urticaceae),and G. binucao (Clusiaceae) with 91.89 and 91.68 mg GAE/ 100 g fw respectively (Figure 2 ). Both L. benguetensis and G. binucao have equal amount of polyphenols present.
R. fraxinifolius is an accumulator of polyphenols [38]. The most widely consumed berries belong to Rosaceae (Rubus sp.) and Ericaceae (Vaccinium sp.) families. The polyphenol content of these berries varies from 30 to 1000 mg/ 100 g. The main polyphenols found in these berries are anthocyanins,ellagitannins and proanthocyanidins [39]. The total phenolic content of Rubus caucasicus (Rosaceae) fruit is 381 mg GAE/ 100 g fw [40]. In addition,Rubus hyrcanus (Rosaceae) fruit contains 414-683.25 mg GAE/ 100 g fw [41]. Thus,Rubus sp. fruits are excellent sources of phenolics (196.98-398.67 mg GAE/ 100 g fw) [42]. P. peruviana (Solanaceae) has the lowest level of polyphenols (21.43 mg GAE/100 g fw) followed by S. pimpinellifolium (Solanaceae) and M. rosacea (Musaceae) with 22.7 and 44.74 mg GAE/100 g fw respectively. Solanaceae fruits have low total phenolic content [37].
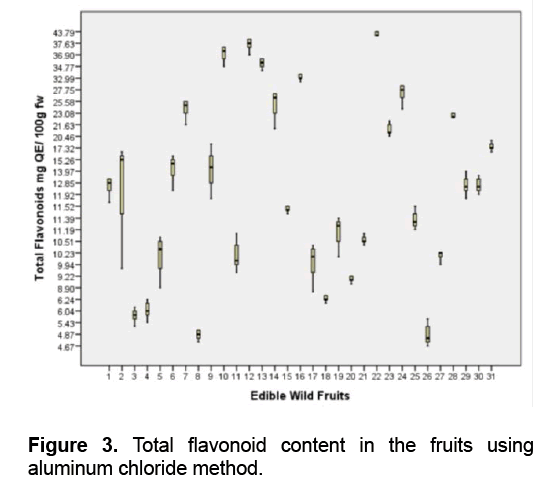
Among the fruits studied,A. montanum (Phyllanthaceae) is the richest source of flavonoids followed by M. pendula (Melastomataceae) and V. myrtoides (Ericaceae) with 41.11,37.41,36.26 mg QE/ 100 g fw respectively (Figure 3 ). Five fruits namely D. philippinensis (Dilleniaceae),P. peruviana (Solanaceae),S. sparsifolia (Actinidiaceae),C. manillensis (Arecaceae) and M. rosacea (Musaceae) are ranked lowest with 4.85,5.11,5.69,5.9 and 6.26 mg QE/ 100 g fw respectively.
A recent study in Kanchanabul province,Thailand reported that Phyllanthus emblica (Phyllanthaceae) fruits contain the highest total phenolics (3703 ± 1244 mg GAE/ 100 g) [43]. The flavonoid content of P. emblica fruit is 143.1 to 148.2 mg catechin equivalence/ 100 g fw [44]. In addition,Antidesma ghaesembilia (Phyllanthaceae) fruit contains high total polyphenol content of 120.818 mg/g GAE and total flavonoid 95.72 mg/g QE [45].
Blueberries (Ericaceae) are also rich sources of flavonoids [46]. The total flavonoid content of Vaccinium myrtillus and Vaccinium vitis-idaeae in 80% methanol is 511.1 mg catechin equivalent/ 100 g dried fruit and 533.6 mg catechin equivalent/ 100 g dried fruit respectively [46]. Compared to the level of polyphenols,the flavonoid content of the fruits is lower. Flavonols are the most ubiquitous flavonoids in foods and the main representatives are quercetin and kaempferol. They are however generally present at relatively low concentrations of 15-30 mg/kg fresh weight [47].
Antioxidant activity of fruits using DPPH assay
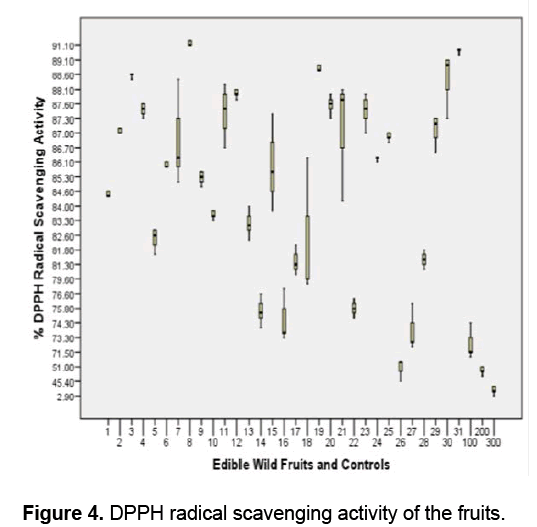
Figure 4 shows that all the fruits exhibited significant scavenging activity against DPPH radicals compared to the positive controls Vitamin E,ascorbic acid and trolox (F value 696.0617 > F crit 1.596293; ρ ≤ 0.05 ). In this study,the ability of fruit extracts and controls to donate hydrogen atom or electron to the unpaired DPPH radical was indicated by the observed change in color of the DPPH solution from purple to yellow [48,49].
D. philippinensis (Dilleniaceae) has the highest antioxidant activity as indicated by its 91.13 % DPPH radical scavenging activity followed by L. haenkei (Zingiberaceae) and P. guajava (Myrtaceae) with 89.6% and 99.93 % respectively.
In a similar study,Dillenia indica (Dilleniaceae) fruit extract exhibited the highest antioxidant activity in methanol followed by ethyl acetate and water [50]. Other studies reveal that there is higher antioxidant activity of edible wild fruits as compared with synthetic vitamin E and ascorbic acid. In Nepal,fifteen fruits commonly used by the ethnic population were studied for the antioxidant activity (Figures 2-4).
Among them,Terminalia bellirica (myrobalan),Terminalia chebula (hardad),Phyllanthus emblica (gooseberry) and Spondias pinnata (mombin) were the most potent antioxidants as compared with vitamin C based on DPPH assay [3]. This is attributed to the total phenolics present in the fruits. They are considered powerful antioxidants in vitro and proven to be more potent anti-oxidants than Vitamin C and E and carotenoids [51]. Many studies have shown that flavonoids and polyphenols are better antioxidants than vitamins [52].
The antioxidant activity between and among the fruits and controls is significantly different (ρ ≤ 0.05) except between and among the following: D. philippinensis,L. haenkei,P. guajava,S. sparsifolia,A. lepicarpum,M. pendula,P. edulis,C. manillensis,F. rukam,P. serratifolia,S. elegans,A. vanoverberghii,S. betacea and A. bunius; G. vidalii,R. fraxinifolius,R. edulis,F. minahassae and V. barandanum; L. benguetensis and M. calabura; A. montanum,F. cumingii,M. alba,S. pimpinellifolium and Vitamin E; P. peruviana and trolox.
Correlation between secondary metabolites,total polyphenol and flavonoid content to antioxidant activity of the fruits
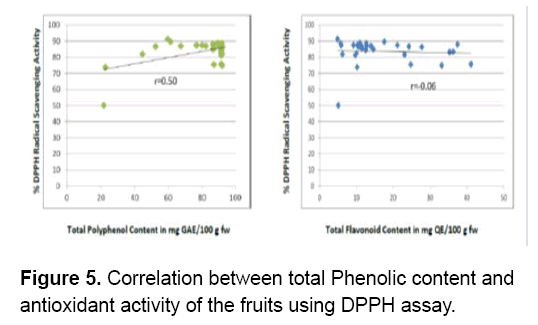
Figure 5 shows that the polyphenol content of the fruits significantly contributed to the antioxidant activity of the fruits (r=0.50). As the concentration of polyphenols increase the antioxidant activity also increases hence,there’s a positive moderate correlation between the two. A variety of polyphenols are found in fruits. Each possesses numerous phenol structures that are responsible for the unique physical,chemical and biological properties of the molecule [53]. Similar results were obtained between the total phenolic content of Elaeagnus angustifolia (oleaster) fruit seeds and its reducing power (r=0.64),between total phenolic content of the peel and its DPPH radical scavenging and (r=0.50) [54]. The antioxidant activity of phenolics is mainly due to their ability to act as reducing agents [55].
Based on the principle of the assay,the presence of hydroxyl groups in the polyphenols reduced DPPH radicals by their ability to donate hydrogen [48]. Aside from these,their low molecular weight contributes to their high scavenging activity on DPPH [56]. Further,polyphenols can scavenge and inactivate reactive oxygen intermediates to prevent oxidative reactions [28].
On the other hand,flavonoids present in the fruits do not significantly contribute to the antioxidant activity of the fruits (r=-0.06). There is a low negative correlation between the two. Therefore,the flavonoids in the fruits do not influence their ability to scavenge DPPH radicals. However,the presence of other secondary metabolites such as alkaloids,saponins and steroids may have contributed to their antioxidant activity.
A negative correlation was observed between the total flavonoid content and antioxidant activities of seven Umbelliferae fruits namely Bunium persicum,Coriandrum sativum,Cuminum cyminum,Foeniculum vulgare,Heracleum persicum,Pimpinella anisum and Trachyspermum copticum from Iran. Flavonoids can act as proton donors however; the position of hydroxyl group on the molecules shall determine their antioxidant properties [57,58]. The results of this study agree with previous researches on the lack of or negative correlation between flavonoids and antioxidant activity [49,59-62].
Aside from polyphenols and flavonoids,other secondary metabolites in the fruits may significantly contribute to the antioxidant activity of the fruits. As inferred from other studies,phenolic compounds in fruits have synergistic action [50]. In the case of D. philippinensis,the low flavonoid content of 4.85 mg QE/ 100 g fw was supplemented by other metabolites present such as alkaloids,steroids,saponins and tannins. The low antioxidant activity of P. peruviana (Solanaceae) may be attributed to the low concentration of polyphenols (21.43 mg GAE/ 100 g fw) and flavonoids (5.11 mg QE/ 100 g fw) in the fruit. The presence of alkaloids,steroids and tannins did not significantly increase its antioxidant activity. Vitamin C and carotenoids are present in the fruit but these have lower antioxidant activity compared to phenolic compounds such as polyphenols [63-64].
Conclusions and Recommendations
The edible wild fruits possess higher or equal antioxidant activity as compared to Vitamin E,C and trolox. Secondary metabolites such as alkaloids,steroid glycosides,saponins and phenolics contribute to their antioxidant activity. The fruits can serve as natural sources of antioxidants. Consumption and domestication of edible wild fruits most especially D. philippinensis must be promoted not only in Benguet but also in the whole country. Isolation,purification,characterization and structural elucidation of polyphenols present in D. philippinensis using High Performance Liquid Chro-matography Mass Spectrometry (HPLC/MS) and Nuclear Magnetic Resonance Spectroscopy (NMR) are recommended for future work.
Acknowledgements
The author is very grateful to the following: Department of Science and Technology (DOST) for the scholarship given and financial grant (D 2009- 15-00464) which was used in this study; provincial governor Hon. Nestor B. Fongwan and mayors of the 13 municipalities in Benguet namely: Hon. Peter B. Alos (Atok),Hon. Marcelo B. Contada (Bakun),Hon. Mauricio T. Macay (Bokod),Hon. Melchor D. Diclas,MD (Buguias),Hon. Oscar M. Camantiles (Itogon),Hon. Faustino M. Aquisan (Kabayan),Hon. Atty. Roberto K. Canuto (Kapangan),Hon. Benito D. Siadto (Kibungan),Hon. Gregorio T. Abalos (La Trinidad),Hon. Atty. Materno R. Luspian (Mankayan),Hon. Arthur C. Baldo (Sablan),Hon. Florencio V. Bentrez (Tuba) and Hon. Atty. Ruben E. Paoad (Tublay) together with the captains,leaders,elders and indigenous peoples in the 140 barangays for their support,assistance and participation in the study; Dr. Gaudelia Reyes,Dr. Evelyn Oda,Dr. Paulina Bawingan,Dr. Lorenza Lirio,Dr. Araceli Ladilad,Dr. Praxedes Rosuman,and Mr. Jonathan Barcelo for their constructive criticisms and suggestions; Mr. Macario Toyan,Ms. Marivic Toyan,Mrs. Esther Bolislis,Mr. Galo Santa,Mr. Jr Santa and Ms. Darlene Tan for their kind assistance in the collection of samples; Dr. Teodora Balangcod,Mr. Dany Tandang,Mr. Isaiah Bautista,Dr. Pieter Pelser,Dr. Julie Barcelona,Ms. Hadassah Chen,Dr. George Argent,Mr. Derek Cabactulan,Ms. Lillian Rodriguez and Ms. Marylou Andrada for their assistance in the identification and verification of plant specimens.
References
- Bounous G,Beccaro GL,Mellano MG,Botta R. (2009). Nutrional value and antioxidant activity of minor fruits grown in Piemonte,Italy. Acta Hortic 818: 249-252.
- Jablonska-Rys E,Zalewska- Korona M,Kalbarczyk J. (2009). Antioxidant capacity,ascorbic acid and phenolic content in wild edible fruits. J Fruit Ornam Plant Res 17: 115-120.
- Chalise JP,Acharya K,Gurung N,et al. (2010). Antioxidant activity and polyphenol content in edible wild fruits from Nepal. Int J Food Sci Nutr 61: 425-432.
- Rawat S,Jugran A,Giri L,Bhatt ID,Rawal RS (2011). Assessment of Antioxidant Properties in Fruits of Myrica esculenta: A Popular Wild Edible Species in Indian Himalayan Region. Evid Based Complement Alternat Med 2011: 1-8.
- Benbrook C. (2005). Elevating antioxidant levels in food through organic farming and food processing. The Organic Center.
- Mazza G,Oomah B. (2000). Herbs,botanicals & tea. Technomic Publishing Co. Inc.,United States,pp. 265-314.
- Valmero A. (2010). DOH says breast cancer is leading cause of cancer deaths in RP. Inquirer.net.
- World Health Organization. (2013). Cancer fact sheet. https://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 10 July 2015
- Reiger M. (2006). Introduction to fruit crops. Haworth Press,New York,pp. 1-50.
- Chattopadhyay S. (2007). Handling,transportation and storage of fruits and vegetables. Tarun Offset Printers Delhi,New Delhi 19-24.
- Martin G. (1995). Ethnobotany. Cambridge University Press,Great Britain 81.
- Mahattanatawee K,Manthey JA,Luzio G,et al. (2006). Total antioxidant activity and fiber content of select Florida-grown tropical fruits. J Agric Food Chem 54: 7355-7363.
- Nobakht G,Kadir M,Stanslas J. (2010). Analysis of preliminary phytochemical screening of Typhonium flagelliforme. Afr J Biotechnol 9:1655-1657.
- Deighton N,Brennan R,Finn C,Davies H. (2000). Antioxidant properties of domesticated and wild Rubus species. J Sci Food Agr 80: 1307-1313.
- Aguinaldo A,Espeso E,Guevara B,Nonato M. (2004). A guidebook to plant screening: phytochemical and biological: botany section. Research Center for the Natural Sciences,University of Santo Tomas,Philippines. 24-50.
- Adedayo B,Oboh G,Akindahunsi A. (2010). Changes in the total phenol content and antioxidant properties of pepperfruit (Dennettia tripetala) with ripening. Afr J Food Sci 4: 403-409.
- Siddique N,Mujeeb M,Najmi A,Akram M. (2010). Evaluation of antioxidant activity,quantitative estimation of phenols and flavonoids in different parts of Aegle marmelos. Afr J Plant Sci 4:1-5.
- Pourmorad F,Hosseinimehr S,Shahabimajd N. (2006). Antioxidant activity,phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol 5:1142-1145.
- Mohy-ud-din A,Khan Z,Ahmad M,et al. (2009). Chemotaxonomic significance of flavonoids in the Solanum nigrum complex. J Chil Chem Soc 54: 486-490.
- Balasundram N,Ai TY,Sambanthamurthi R,Sundram K,Samman S. (2005). Antioxidant properties of palm fruit extracts. Asia Pac J Clin Nutr 14: 319-324.
- Devi KP,Suganthy N,Kesika P,Pandian SK. (2008). Bioprotective properties of seaweeds: in vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement Altern Med. 8: 38.
- Erasto P,Mbwambo Z. (2009). Antioxidant activity and HPTLC profile of Lagenaria siceraria fruits. Tanzan J Health Res. 11:79-83.
- Cantoria M. (1994). Selected topics in pharmacognosy. APO Production Unit,Inc.,Philippines,p. 371.
- Herraiz T,Galisteo J. (2003). Tetrahydro-beta-carboline alkaloids occur in fruits and fruit juices. Activity as antioxidants and radical scavengers. J Agric Food Chem. 51: 7156-7161.
- Koduru S,Jimoh F,Grierson D,Afolayan A. (2007). Antioxidant activity of two steroid alkaloids extracted from Solanum aculeastrum. JPT. 2: 160-167.
- Yucekutlu A,Bildaci I. (2008). Determination of plant saponins and some Gypsophila species: a review of the literature. Hacettepe Journal of Biology and Chemistry. 36:129-135.
- Elekofehinti O,Adanlawo I,Komolafe K,Ejelonu O. (2012). Saponins from Solanum anguivi fruits exhibit antioxidant potential in Wistar rats. Ann Biol Res. 3: 3212-3217.
- Smirnoff N. (2005). Antioxidants and reactive oxygen species in plants. Blackwell Publishing,India. 152.
- Chodak A,Tarko T. (2007). Antioxidant properties of different fruit seeds and peels. Acta Sci Pol Technol Aliment. 6: 29-36.
- Zhang LL,Lin YM. (2008). Tannins from Canarium album with potent antioxidant activity. J Zhejiang Univ Sci B. 9: 407-415.
- Prior RL,Wu X,Schaich K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 53: 4290-4302.
- Matthes A,Eiberger M. (2009). Polyphenol content and antioxidant capacity of apple fruit: effect of cultivar and storage conditions. J Appl Bot Food Qual. 82: 152-157.
- Uzelac V,Kovacevic D,Levaj B,et al. (2009). Polyphenols and antioxidant capacity in fruits and vegetables common in the Croatian diet. Agric Conspec Sci. 74: 175-179.
- Hossain M,Rahman S. (2011).Total phenolics,flavonoids and antioxidant activity of tropical fruit pineapple. Food Res Int. 44: 672-676.
- Kumarappan CT,Thilagam E,Mandal SC. (2012). Antioxidant activity of polyphenolic extracts of Ichnocarpus frutescens. Saudi J Biol Sci. 19: 349-355.
- Biesiada A,Tomczak A. (2012). Biotic and abiotic factors affecting the content of the chosen antioxidant compounds in vegetables. Veg Crop Res. 76:55-78.
- Vasco C. (2009). Phenolic compounds in Ecuadorian Fruits. Dissertation,Swedish University of Agricultural Science,SLU Service,Uppsala. 12-47.
- Flora Malesiana. (1993). Spermatophyta: Rosaceae,Amaryllidaceae,Alliaceae,Coriariaceae,Pentastemonaceae,Stemonaceae.
- Neveu V,Perez-Jiménez J,Vos F,et al. (2010). Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010. bap024.
- Yildiz H,Segul M,Celik F,et al. (2010). Some phytochemical and antioxidant characteristics of wild and cultivated blackberry (Rubus caucasicus) fruits. J Food Agric Environ. 8: 156-159.
- Kivi A,Nejad R,Dehgan G,Najafi F,Hajilo J. (2013). Antioxidant capacity and phytochemical properties of raspberry species in Iran. Int J Biosci. 3: 145-152.
- Botta R,Contessa C,Mellano M,Beccaro G. (2013). Total antioxidant capacity and total phenolic and anthocyanin contents in fruit species grown in Northwest Italy. Sci Hortic. 160: 351-357.
- Judprasong K,Charoenkiatkul S,Thiyajai P,Sukprasansap M. (2013). Nutrients and bioactive compounds of Thai indigenous fruits. Food Chem. 140: 507-512.
- Ruangchakpet A. Sajjaanantakul T. (2007). Effect of browning on total phenolic,flavonoid content and antioxidant activity in Indian gooseberry. Witthayasan Kasetsat. 41: 331-337.
- Habib M,Rahman M,Hamid K,Raihan M,Sayeed M. (2011). Phytochemical screening ,cytotoxicity,antioxidant capacity and antibacterial potentiality of methanol extract of Antidesma ghaesembilla. Adv Nat Sci. 5: 69-74.
- Manach C,Scalbert A,Morand C,Rémésy C,Jiménez L. (2004). Polyphenols: food sources and bioavailability. Am J Clin Nutr. 79: 727-747.
- Pop M,Lupea A,Turcus V. (2008). Studies on the polyphenolic compounds extraction from Vaccinium fruits. Rev Chem Bucharest. 59: 491-494.
- Nurhanan A,Rosli W,Mohsin S. (2012). Total polyphenol content and free radical scavenging activity of cornsilk (Zea mays hairs). Sains Malays. 41: 1217-1221.
- Zulkifli K,Abdullah N,Abdullah A,Aziman N,Kamarudin W. (2012). Phytochemical screening and activities of hydrophilic and lipophilic antioxidant of some fruit peels. MJAS. 16: 309-317.
- Abdille M,Singh R,Jayaprakasha G,Jena B (2001) Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem. 90: 891-896.
- Rice-Evans C,Miller N,Paganga G. (1997). Antioxidant properties of phenolic compounds. Trends Plant Sci. 2: 152-159.
- Pietta PG. (2000). Flavonoids as antioxidants. J Nat Prod. 63: 1035-1042.
- Quideau S,Deffieux D,Douat-Casassus C,Pouvsequ L (2011). Plant polyphenols: chemical properties,biological activities,and synthesis. Angewandte Chemie International Edition. 50: 586-621.
- Faramarz S,Dehghan G,Jahanban-Esfahlan A. (2015). Antioxidants in different parts of oleaster as a function of genotype. Bioimpacts. 5: 79-85.
- Kähkönen MP,Hopia A,Vuorela HJ,et al. (1999). Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 47: 3954-3962.
- Othman A,Muktar N,Ismail N,Chang S. (2014). Phenolics,flavonoid content and antioxidant activities of four Malaysian herbal plants. Food Res Int. 21:759-766.
- Nickavar B,Abolhasani FA. (2009). Screening of antioxidant properties of seven Umbelliferae fruits from Iran. Pak J Pharm Sci. 22: 30-35.
- Miliauskas G,Venskutonis P,Van Beek T. (2004). Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 85: 231-237.
- Heinonen IM,Lehtonen PJ,Hopia AI. (1998). Antioxidant Activity of Berry and Fruit Wines and Liquors. J Agric Food Chem. 46: 25-31.
- Hajilou J,Fakhimrezaei S,Dehghan G. (2013). Fruit quality,bioactive compounds and antioxidant capacity of six Iranian peach cultivars. RPB. 3: 6-16.
- Fidrianny I,Windyaswari A,Wirasutisna K. (2013). DPPH scavenging activity of various extracts of sweet potatoes leaves with varying tuber colors. IJRPS. 3: 133-145.
- Matthäus B. (2002). Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem. 50: 3444-3452.
- Valdenegro M,Henriquez C,Lutz M,Almonacid S,Simpson R. (2010). Drum dried,lyophilized dried and traditional drying of goldenberry (Physalis peruviana): effect in nutritional and healthy quality. Proceedings of the International Conference on Food Innovation Food Innova,Valencia,California,USA
- Gil MI,Tomás-Barberán FA,Hess-Pierce B,Kader AA. (2002). Antioxidant capacities,phenolic compounds,carotenoids,and vitamin C contents of nectarine,peach,and plum cultivars from California. J Agric Food Chem. 50: 4976-4982.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences