Kinetic Study of the Growth of Saccharomyces Cerevisiae from Date Syrup Variety HMIRA
Hariri A, Ouis N, Bouhadi D
1 Bioconversion Laboratory, Microbiology Engineering and Health Safety, Department of Biology, Faculty of Science the Nature and Life, University of Mascara, (UN 2901), BP.763, Sidi Said, Mascara, 29000, Algeria
2 Laboratory of Chemisty, Department of Chemistry, Faculty of Sciences, University of Oran Es- Sénia, BP 1524 El M’naouer, Oran, 31100, Algeria
Received date: January 27, 2016; Accepted date: February 08, 2016; Published date: February 14, 2016
Citation: Hariri A, Ouis N, Bouhadi D, Kinetic Study of the Growth of Saccharomyces cerevisiae from Date Syrup Variety HMIRA. Electronic J Biol, 12:2
Abstract
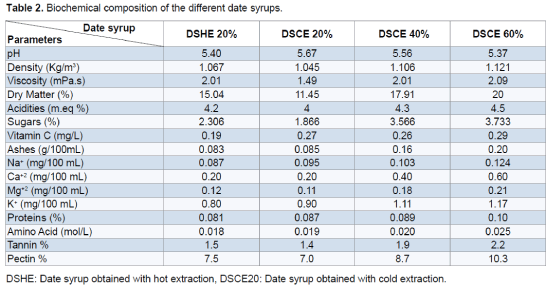
The objective of this study is the use of date syrup varieties Hmira like substrate for the growth of Saccharomyces cerevisiae. The study of the optimization of method extraction show that the mode of hot extraction increases the concentrations of sugars, dry matter, tannins and pectin relative to the cold extraction which promotes the extraction of vitamin C and proteins. The different physicochemical and biochemical analyzes show the richness of date syrup in nutritional elements that makes them favorable to the alcohol fermentation. Kinetic study of the growth of Saccharomyces cerevisiae shows that perfect adaptation of strain to the medium (date syrup) and the concentration of 60% of date syrup is favouring to the growth of this strain.
Keywords
Date syrup; Extraction; Saccharomyces cerevisiae; Fermentation; Alcohol.
1. Introduction
Nature offers to us several raw materials, with real nutritional values. The dates (Phoenix dactylifera L.) are an example with their great content of sugar and dietary minerals. This fruit is appreciated for its taste and its nutritional value [1]. The dates constitute almost the principal resource of the populations in the desert oases. The date has always been a very important part of food for both humans and animals. The number of date palm is estimated today at more than ten million placing Algeria as the fifth producer of the date in the world [2]. The Algerian palm plantation lodges a very rich and diversified genetic material with more than 13 million palm trees and 940 cultivars listed [3]. Algeria is among the largest producers and exporters of dates, with an average production of 473 000 tons which 60 000 to 75 000 tons is minus appreciated on the market, constituted of common of dates and offal’s of Deglet-Nour. The "Deglet Nour" variety is in first place, representing 46.23%, followed by 'Deglet Beidha "with 32.9% of the total production date [4,5]. The dates destined to the local consumption and to the export has morphological, microbiological and physicochemical characteristics well-defined. In parallel, the other varieties known as common are less appreciated, less marketed and less competitive; they represent approximately 30% of the national production [6,7]. The large amount of date is excluded from the human food because of its biological and mechanical alteration among the variety found HMIRA. In contrast, they are very rich in nutriments: sugars, vitamins and minerals [2,5]. The waste and un-wanted materials usually issued from fermentation one being used to produce, protein and carbohydrate. The valorization of common dates by the biotechnical processes represents a solution of choice insofar as it allows producing some substances to high added value.
Bioethanol as an alternative source of energy has received special attention worldwide due to depletion of fossil fuels. Ethanol is a clear, colourless, flammable, oxygenated hydrocarbon with the chemical formula C2H5OH. Ethanol has been made since ancient times by fermenting sugars [8]. By elsewhere, he exists in Algeria two farmers of production of bakery yeast using more than 20000 tons of molasses by year [9]. The molasses is the main raw material for ethanol production but now the short supply and increased cost is the main hindrance for its use. In recent years because of increase in price of molasses and its limited availability, ethanol production has been greatly affected [10]. The utilization of the molasses of beet or of cane could cause some constraints because they can contain some inhibiting of fermentation such as pesticide used during the culture of beets or of cane [11,12]. Under such circumstances a novel approach is essential to use easily available renewable substrates such as date. The sugary substrates available are comparatively expensive than molasses but can be easily used for ethanol production. Alcoholic fermentation is the main activity of yeasts, while Saccharomyces cerevisiae is the major species used in wine making. It utilizes sucrose, glucose, fructose, and maltose and maltotrioses carbon sources to produce alcohol under anaerobic conditions. Indeed, this date is rich in sugars that could be used as carbonaceous source of fermentation for the production of the biomass and ethanol. The objective of this study is the utilization of the date varieties HMIRA as the substrate for the growth of Saccharomyces cerevisiae and the production of ethanol.
2. Material and Methods
2.1 Material
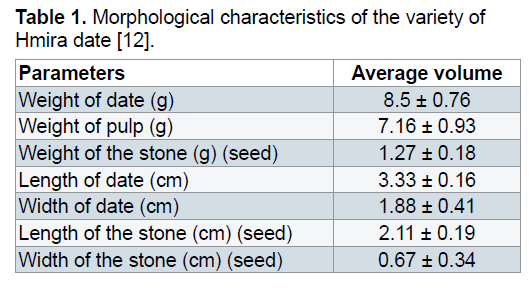
Vegetable material: The date (Phoenix dactylifera L.) used in current experiments was a variety half soft, known as HMIRA badly exploited, collected in the month of June 2012 from the region of Bechar (South-west of Algeria). The choice of this variety is justified by their availability and their important nutritive value, especially of reducing sugars. Its characteristics are given in Table 1.
Biologic material: The material used is pure yeast Saccharomyces cerevisiae derived from the industrial bakery yeast (Oued Smar-Algiers, Algeria).
2.2 Methods
Preparation of date syrup: The dates were washed in order to eliminate sand and dust, and then cut into the small pieces to increase the surface of diffusion. Five liters of water were added to 1 kg of date, homogenized and through a cloth. The extraction parameters were obtained from method advocated by Turhan et al. [13]. The pieces of dates were mixed with distilled water at a concentration 20% (200 g of dates in 1 L of water) and left during 2 h at 90°C. For the cold extraction, the pieces of dates were mixed with distilled water at concentrations of 20, 40 and 60% and left during 24 h at 30°C. The mixture was filtrated to resound the large particles, after filtration the syrup was subjected a centrifugation of 5000 rpm during 30 min, then sterilization in the autoclave at 120°C during 15 min under a maximum pressure of 2 bars has been applied. The syrup collected is characterized by a honey colour, a pleasant odour and very sweetened.
Preparation of the inoculums: A stock culture of Saccharomyces cerevisiae was maintained on agar media slopes which were stored in screw-cap bottles at 4°C. The agar medium consists of the following nutrients: Yeast extracts 0.3 g, malt extract: 0.3 g, peptone: 0.5 g, glucose: 1 g, agar: 2 g, distilled water 100 ml. After transplanting, the strains were incubated at 30°C for 72 h and then preserved at 4°C. Sub-culturing was carried out at intervals of one month. The inoculums were prepared by transferring yeast cells from slant of 250 mL of Carlsberg medium. The flasks were subjected to the orbital previously adjusted at 30°C and 150 rpm and incubated for 18 h [14].
Conduction of the alcoholic fermentation: Seeding of the yeast Saccharomyces cerevisiae is performed at a dose of 1%. After preliminary testing of 0.5 to 5%, a low dose gives a very slow fermentation and a high dose causes foaming and gas that interferes with the control and followed by fermentation. The fermentation of these syrups was conducted under the following conditions: temperature 30°C, initial concentration of inoculation (1 g yeast in 100 mL of syrup), initial pH and the initial concentration of reducing sugars in the syrup. The fermentation is conducted in two phases: the first allows adapting the strain Saccharomyces cerevisiae to the medium to be studied and the second is used to inoculate another volume of above medium with the resulting suspension by the first step [15].
• First step: We took a series of four 250 mL flasks, each containing 10 mL of date syrup, 1 g of yeast and mix thoroughly until no more lumps.
• Second Step: We added 90 mL of date syrup to each flask and closed them carefully to ensure anaerobically. At the same time it took 4 flasks each containing 25 mL of Ba(OH)2 0.5 N, a few drops of phenolphthalein and we realized the assembly shown in Figure 1 to a temperature of 30°C and maintaining agitation. We followed the evolution of the concentration of products (ethanol, CO2), biomass, that of the nutrient concentration (sugars), and the physicochemical parameters (pH, temperature and conductivity) every hour for 5 h [15].
Analytical methods
• Physicochemical analysis: The pH and conductivity are measured using a pH meter. The density was determined by density meter [16] and the viscosity is measured using the drop bile viscometer (HAAKE). The optical density is measured by spectrophotometer at 420 nm of filtered juice [17].
• Biochemical analysis: The concentration of lactic acid was determined by acidity titration with NaOH 0.1 N [18,19]. The total nitrogen and content proteins are determined by the method of Kjedahl [20-22]. The reducing sugars, the sucrose and the total sugars were determined by Dubois method [23]. The content in water and dry matter are determined by drying 10 mL of syrup at 105°C during 18h [18,24-26]. The content of vitamin C is determined according to the methods advocated by [27].The content in ashes is determined by incineration one gram of syrup at a temperature of 600°C during 3 h [18,24,25] and the mineral salts are determined according to the methods advocated [21,28].
• Determination of amino acid content: For 5 mL of date syrup and 1 mL of ninhydrin reagent then the mixture was heated at 80-160°C during 4 minutes. The optical density of control and the sample were measured at 570 nm. In the control, we replace the syrup with distilled water. At the same time we realize a calibration with different concentrations of glycine.
• Determination of tannin content [22]: With 6 mL of butanoic acid and 1 mL of date syrup was added with stirring 0.2 mL of reactive iron and then put the mixture in boiling water bath for 50 minutes. The absorbance of the control and the sample are measured at 550 nm. In the control, we replace the syrup with distilled water. At the same time, we make the standard curve with different concentrations of tannic acid.
• Determination of pectin [22]: To 10 g of date syrup was added with stirring 10 mL of 10% NaOH and then after standing for 5 minutes introducing 4-8 mL of HCl 5 N followed by heating for 5 min. After filtration, the capsule is placed in an oven at 105°C until a constant weight (P1). After drying, it is introduced in a muffle furnace at 700°C; we conducted a further weighed (P2). The percentage of pectin is given by the following formula:
• Where Pectin % = (P1–P2) / M.10, P1: The sample mass after drying, P2: The sample mass after calcinations, M: The mass of the test.
• CO2 production [15]: CO2 is trapped by the Ba(OH)2 and gives BaCO3 (barium carbonate), which remains the Ba(OH)2 is titrated with oxalic acid to precipitate and does not destroy the barium carbonate. Every hour, we took a flask containing the Ba(OH)2 and titrated using a oxalic acid 0.5N until the inductor color disappears. Each milliliter of titrate represents:
• 44 × 0.1/2 × 1000.
• CO2 (mg) = (v1 - v2) × N × 44/2 × 1000
• Where v1: the volume of oxalic acid which reacts with 25 mL of Ba(OH)2, v2: the volume of oxalic acid which reacts with the rest of Ba(OH)2 after fermentation.
• Biomass production: The concentration of the biomass is determined by measuring the turbidity of syrup every hour using a turbidity meter that gives a direct reading. At the same time, we make the calibration of different concentrations of yeast.
• Ethanol production: Since the concentration of ethanol is a small quantity, it is measured for 1, 3 and 5 hours of culture. It took three flasks and each was placed in 500 mL of date syrup and 5 g of yeast Saccharomyces cerevisiae. The flask is closed tightly to ensure anaerobiosis and then we did the reading concentration of alcohol with alcoholmeter "Gay Lussac".
• Volumetric rate of biomass, CO2 production and sugars consumption: Volumetric rate of biomass production (Vx), CO2 production (Vp) and sugars consumption (Vs) are calculated by the following equations:
V × (g/L.h) = (Xf -Xo) / Dt
Where Dt: fermentation time in hours, Xo and Xf: initial and final concentration of biomass
Vp (μmol/L.h) = (Pf-Po) / Dt
Where Po and Pf: initial and final concentration of product.
Vs (%/L.h) = (So-Sf) / Dt
Where So and Sf: initial and final concentration of sugar consumed.
3. Results and Discussion
3.1 Results of physicochemical and biochemical analyzes of date syrup
The obtained result shows that the date syrup presents acidities between 4 and 4.5 and pH ranging from 5.37 to 5.67. These pH values are favorable for the growth of yeast. Similar pH values (5.9) were obtained by Bouhadi et al. [12] and Kaidi and Touzi [29]. It seems that the mode of hot extraction is benefit increases the amount of sugars extracted (23.06 g/L), dry matter (15.04%), tannins (1.5%) and pectin (7.5%) relative to the cold extraction user to 20% which promotes the extraction of vitamin C (0.19 mg/L) and proteins (0.081%). The different physicochemical and biochemical analyzes show the richness of date syrup in nutritional elements that makes them favorable to the alcohol fermentation (Table 2).
3.2. Results of the alcoholic fermentation
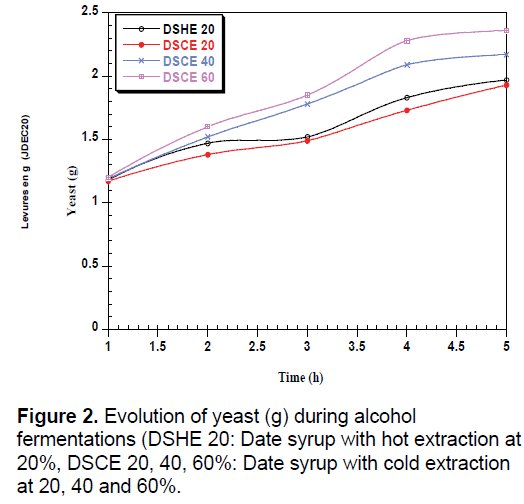
During the fermentation of yeast, there is a total absence of the lag phase for all date syrups used (Figure 2). The time of adaptation of Saccharomyces cerevisiae depend on the strain used and the composition of medium [30,31]. The Work made by Ouldelhadj et al. [32] has shown the time of adaptation of Saccharomyces cerevisiae varies between 2.5 to 2.3 hours.
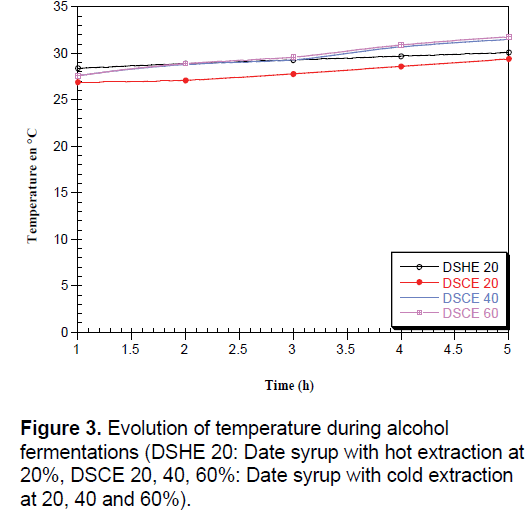
According to Brémond [33], during the fermentative metabolism, the breakdown of sugars into alcohol and carbon dioxide is a reaction exothermic which justified the increase in temperature (Figure 3). The ethanol yield increased due to the increasing temperature from 25°C to 30°C with the inoculation of both Saccharomyces cerevisiae [8].
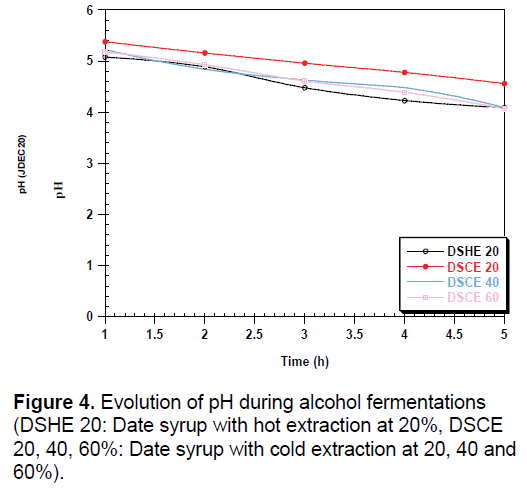
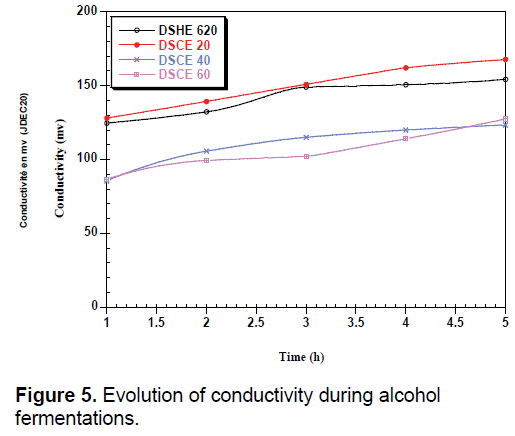
During fermentation, we observed a low decrease in pH can be attributed to the activity of the yeast (Figure 4). The amount of yeast depends on the strain used and the conditions of culture including pH, temperature and agitation [34]. The increase in conductivity during fermentation may be related to the release of minerals that were attached to certain constituents; they are used by yeast for their metabolism (Figure 5).
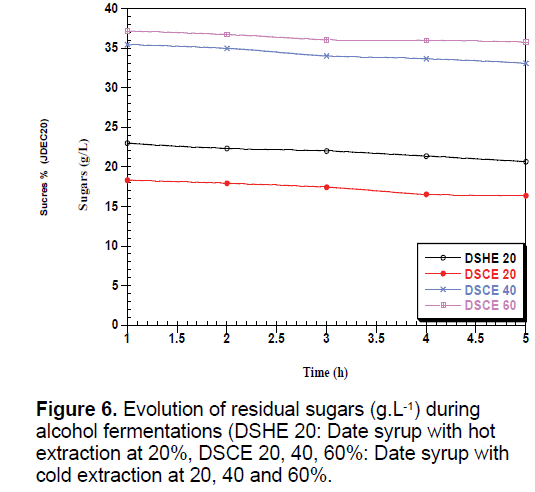
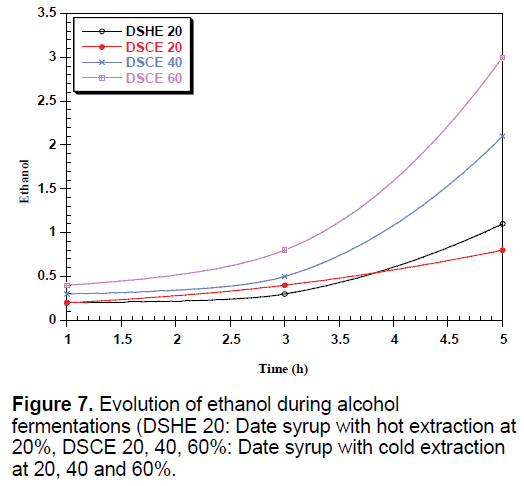
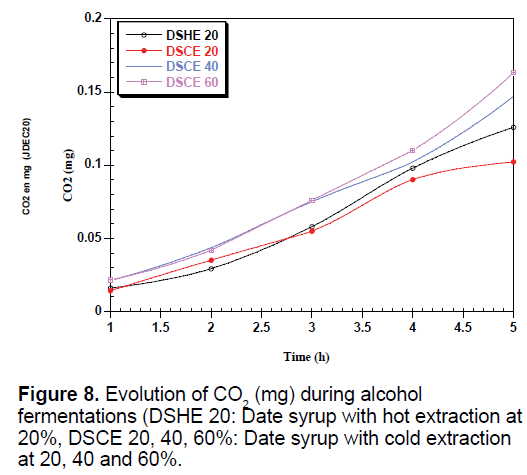
The yeast used sequentially fermentable sugars present in the date syrup, sucrose is hydrolyzed into glucose and fructose by enzyme located in the outer surface of yeast [35]. These sugars are consumed simultaneously converted into biomass, ethanol and CO2, which justify the presence and the increase of these metabolites in all types of date syrup fermentations (Figures 6-8).
Date syrup to 60% advantage promotes fermentation activity of yeasts and production of various metabolic substances. The ethanol production is much higher with increasing concentration. Sasson, reports that fatty acids, especially octanoic acid and decanoic acid, formed by the yeast become toxic to yeast [36].
3.3. Volumetric rate of biomass, CO2 and sugars
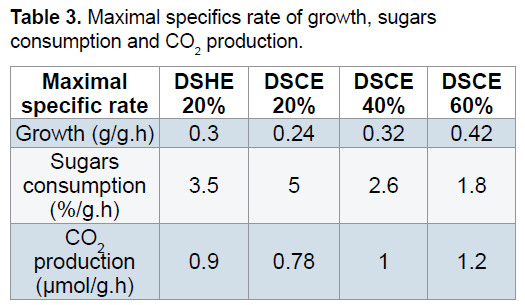
The Increased of the syrup date concentration favored the production of yeast by the presence of sugars and growth factors. The volumetric rate of growth increase to 0.24 g/g.h in syrup extracted with cold at 20% to 0.32 g/g.h in 40% and up to that of 0.42 g/g.h for 60%. Date syrup obtained with hot extraction at 20% enriched advantage the nutritional composition of syrup which explains the volumetric rate of growth recorded 0.3 g/g.h compared to the syrup at 20% obtained with cold extraction. Similar remarks are found to the volumetric rate of CO2 (Table 3). Belin cited by Botton et al. [37] notes that the Saccharomyces cerevisiae growth rate grown on favorable medium between 0.3 and 0.47 h-1. Benabdessalam [38] notes that yeast where high cell densities and maximum conversion of cellular material substrate are desired for economic reasons the specific growth rate is between 0.05 to 0.3 h-1. For the generation time is the time required for doubling the population, it is inversely proportional to the growth rate. So the higher the concentration ratio, the greater the generation time is low or reduced.
4. Conclusion
Ethanol has attracted worldwide interest as a renewable energy source with high performance and low environmental impact motor fuel. Ethanol is a renewable energy source because it is produced via the microbial fermentation by using agricultural based carbohydrates such as starch, sugar, or cellulose with microorganisms such as Saccharomyces cerevisiae (S. cerevisiae, Zymomonas mobilis and Z. mobilis). The molasses is the main raw material for ethanol production but now the short supply and increased cost is the main hindrance for its use. In recent years because of increase in price of molasses and its limited availability, ethanol production has been greatly affected. The utilization of the molasses of beet or of cane could cause some constraints because they can contain some inhibiting of fermentation such as pesticide used during the culture of beets or of cane.
The date substrates available are comparatively expensive than molasses but can be easily used for ethanol production. The objective of this study was the utilization of the date varieties Hmira as the substrate for the growth of Saccharomyces cerevisiae. The different physicochemical and biochemical analyzes showed the richness of date syrup in nutritional elements that makes them favorable to the alcohol fermentation. The results showed the perfect adaptation of Saccharomyces cerevisiae to date medium. The syrup made from dates is rich in sugars and growth factors, environment suitable for culturing Saccharomyces cerevisiae for the production of baker's yeast. The possibility of substitution of dates to molasses seen producing yeast is possible.
References
- AbouZeid AA, Khoja Samir M. (1993). Utilisation of date in the fermentative formation of Citric Acid by Yarrowialipolytica. Zentralblatt fur Mikrobiol.148: 213-221.
- Messar M. (1996). TheAlgerianphoenicicolsector:Situation and Prospects2010.MediterraneanOptions, seriesNo. 28,MediterraneanseminarCIHEMandEstacionphoenix.23-44.
- Hanachi S, Khitri D, Benkhalifa A. (1998).Brac de Perrière RA. Varietalinventoryof the Algerianpalm. 225.
- Boughnou N. (1988). Vinegarproduction testfrom dateswaste. Magisterthesis, NationalInstituteof Agronomy, ElHarrach.
- Anonymous. (1992). Converting and processingof datesand by-productsof dates.Date Symposium. 24-25.
- Anonymous. (2001). Agricultural Statistics, Series A, acreage and production. M.A, D.S.A. E.E ed. 5-6. Ministry of Agriculture, Algiers, Algeria.
- FAO. (2008). Chemical composition offruitsof15 Tunisiancultivarsofdate palm. Biodiversity International.148:19-25.
- Sivasakthivelan P, Saranraj P,Sivasakthi S. (2014).Production of Ethanol by Zymomonasmobilisand Saccharomyces cerevisiaeUsing Sunflower Head Wastes - A Comparative Study. Int J of Microbiological Res.5: 208-216.
- Anonymous. (2002). Manufactureofbaker's yeast: yeast factoryinOuedSmar, Yeast factoryAlgiers,Algeria. 1-20.
- Verma G, Nigam P, Singh D, Chaudary K. (2000). Bioconversion of starch to ethanol in a single step process by coculture of amylolytic yeast and S. cerevisiae. Bioresource Technology.72: 261-266.
- Bouix MY, Leveau JY. (1993). Yeastinindustrial microbiology. APRIA, Paris, France. 1-93
- Bouhadi D, Abbouni B, Hariri A, Ibri k, OuisNawel. (2012). Study of the Behaviour of Lactobacillus delbrueckii subsp. bulgaricus in Date Syrup in Batch Fermentation with Controlled pH.J Biotechnol Biomaterial.2: 1-5.
- Turhan I, Demirci A, Karhan M. (2008). Ethanol production from carob extract by Saccharomyces cerevisiae. ASABE Paper No. NABEC 08–009. St. Joseph, MI: American Society of Agricultural Engineers.
- Al-Obaidi AM, Alhakkak THS, Al-Hilli MA. (1987). Optimization of propagation medium for Baker’s Yeast using date extract and molasses. Determination of the optimum concentration of microelements and vitamins. Date Palm. Jou. 9: 65-78.
- Salman Y. (1984). Plant physiology,practical part, universitySyria, TishreenInstituteof agronomy.91-93.
- Barkatov J, Elissev H. (1979). Guideof practical workof thetechnical-chemical controlof the production ofpreserves.Boumerdes. ENAJUCRelizane.
- Meydav S, Saguy I, Kopelmani J. (1977). Browning determination in citrus products, Agric. Food Chem. 25: 602.
- A.O.A.C. (1970). Officinal methods of analysis. D.C. ed. Ttth. Washington, USA. 5-56.
- Afnor. (1986). Collection ofFrenchstandards, products derived fromfruitsand vegetables.
- Audigie CI, Figarella J, Zonszani F. (1984). Manipulationofbiochemicalanalyzes.Doin. Doinpublishers,Paris, France.88-97.
- Hamon M, Pellerin F, Guenet M, Maauzier G. (1990).Speedanalytical chemistry,spectralmethodsandorganicanalysis.3.2nd edition. Masson.Paris.232-233.
- Multon JL. (1991). Analysis techniquesandcontrols infood industries.4, 2nd edition, TecandDoc.
- Dubois MK. (1956). Colorimetric Method for Determination of Sugar and Related Substances. Anal and Chem. Jour.28: 350.
- Novel G. (1993). Lactic acid bacteriainIndustrial Microbiology, Microorganismsof industrial interest.Ed. Lavoisier. Paris.171-215.
- Salgarolo P. (2003). Practicalchemistrymanipulationswith the use ofBiology,EdLavoisier,Paris6.2003.
- Audigie CL, Dupont G, Zonszain F. (1983). PrinciplesofBiochemicalmethods.EdDoin, T.2, Paris.144.
- Hoffman F. (1971). Vitamincompendium. Ed. rock andcie, S. A.Ball, Switzerland.84-85.
- Godon B, Loisel W. (1997). AnalysisHandbook forthe2nd editioncerealsindustries,TecandDoc, Lavoisier,Paris. 307-331.
- Kaidi F, Touzi A. (2001). Productionofbio-alcohol from to WasteDates. Rev.Energ. Ren.Production andDevelopment-Biomass.75-78
- Monod J. (1942). Researchon growth ofbacterial cultures.EdHermanet Cie,Paris.210.
- Lambin S, German A. (1969). Microbiologyaccurate.EdMassonet Cie,Paris.30-100.
- Ouldehadj M, Bitour Z, Siboukeur O. (2006). Product engineering of yeast baker (Saccharomyces cerevisiae) cultivated on must of dates rejects. Courrier du Savoir. 13-18.
- Brémond E. (1965). Modern winemakingtechniques andwine conservationinMediterranean countries, 2nd edition, Paris.
- Girard H, Rougieux R. (1991). Agriculturalmicrobiologytechniques.EdDunod,Paris: 1958;80-90.
- Larpent JP. (1991). Biotechnologyyeast,Masson,Paris.237-242.
- Sasson A. (1986). Feedingtomorrowmen.EdUnesco, Netherlands.765.
- Boton B, Breton M, Fevre S, et al. (1985). Biotechnologies,useful and harmfulmoldsindustrial importance.EdMasson,Paris. 365-383.
- Benabdessalam A. (1975). Optimal productionofyeastfromlocalmolasses.PhDEng.,INAElHarrach.52.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences