Isolation and Screening of Aspergillusspp.for Pectinolytic Activity
Anisa S.K., Ashwini S., Girish K
Postgraduate Department of Microbiology, Maharani's Science College for Women, JLB Road, Mysore - 570 005, Karnataka, India
- Corresponding Author:
- Tel: +91-9743665772
E-mail: girishk77@yahoo.com
Abstract
Pectinolytic enzymes are one of the several extracellular enzymes produced by fungi that break down pectin, a polysaccharide substrate that is found in the cell walls of plants. Preparations containing pectin-degrading enzymes have been extensively used in the clarification of fruit juices and wines. Currently, they are widely used in industry for retting of natural fibers and extraction of oils from vegetable and citrus peels. In the present investigation, several Aspergillus spp., isolated from agricultural waste, leaf litter, farmyard manure and cow dung on pectin agar medium viz., Aspergillus flavus, A. niger, A. Ochraceous, and Aspergillus sp., were tested for pectinolytic activity by plate assay, turbidity assay, turbidometric assay and polygalacturonase assay. Zone of clearance and clearance of turbidity observed in plate assay and turbidity assay respectively showed the production of pectinolytic enzymes by these fungi. The turbidometric assay and polygalactorunic assay clearly indicated the ability of these fungi to produce pectinolytic enzymes, especially the polygalacturonase enzyme.
Keywords
Aspergillus spp., extracellular enzymes, pectinolytic enzymes, po1ygalacturonase
1. Introduction
Pectin is a structural heteropolysaccharide contained in the primary cell walls of terrestrial plants. It was first isolated and described in 1825 by Henri Braconnot [1]. During ripening, pectin is broken down by the enzymes pectinase and pectin esterase; in this process the fruit becomes softer as the middle lamella breaks down and cells become separated from each other. Pectinase is a general term for enzymes, such as pectolyase, pectozyme and polygalacturonase, commonly referred as pectic enzymes [2]. These break down pectin, a polysaccharide substrate that is found in the cell walls of plants. One of the most studied and widely used commercial pectinase is polygalacturonase [3]. Pectic enzymes are commonly used in processes involving the degradation of plant materials, such as speeding up the extraction of fruit juice from fruits.
Pectinolytic enzymes are widely used in the food industry and in wine production [4]. The function of pectinase in brewing is two-fold, first it helps to breakdown the plant (typically fruit) material and so helps the extraction of flavors from the mash. Secondly the presence of pectin in finished wine causes a haze or slight cloudiness, pectinase is used to break this down and so clear the wine [3]. Pectinases are also used for retting. Therefore there is a huge demand for pectinases in industries. They have a share of 25% in the global sales of food enzymes [5].
Microbial pectolysis is important in plant pathogenesis, symbiosis and decomposition of plant deposits [6]. Pectolytic enzymes are wide spread in nature and are produced by bacteria, fungi, protozoa and nematodes [2]. Fungi produce several extracellular enzymes that result in the decomposition of organic matter. One such enzyme is pectinolytic enzymes. The fungus produces these enzymes to break down the middle lamella in plants so that it can extract nutrients from the plant tissues and insert fungal hyphae [7,8]. The members of the fungal genus Aspergillus are commonly used for the production of polysaccharide degrading enzymes. This genus produces a wide spectrum of cell wall degrading enzymes, allowing not only complete degradation of the polysaccharide but also tailored modification by using specific enzymes purified from these fungi [9]. In this study an attempt has been made to isolate and screen Aspergillus spp., from different sources for their ability to produce pectinolytic enzymes.
2. Materials and Methods
2.1 Isolation of fungi
Isolation of pectin degrading fungi was done from agricultural waste, leaf litter, farmyard manure and cow dung by serial dilution method on pectin agar media following standard procedures. The plates were incubated at room temperature for about 3 to 5 days. The plates were observed for the growth of fungal colonies after incubation period and pure cultured.
2.2 Plate assay of depolymerized pectin
Plate assay was carried out by inoculating pectin agar medium having 1% pectin with pure cultures of fungi (A. flavus, A. niger, A. Ochraceous, and Aspergillus sp.) separately. The inoculated plates were incubated for about 72 hours at room temperature. After the colonies reached around 3 to 4 mm iodine potassium iodide solution was added to detect the clear zone [10].
2.3 Turbidity assay
Turbidity assay was carried out using pectin broth medium with 1% pectin and pure cultures of fungi (A. flavus, A. niger, A. ochraceous and Aspergillus sp.). Sterile pectin broth medium in test tubes were inoculated with 1 ml of spore suspension of A. flavus, A. niger A. ochreceous and Aspergillus sp., separately. A tube inoculated with 1.0 ml distilled water was maintained as control. All the tubes were incubated at room temperature for about 5 days. Change in turbidity was observed by comparing with control tube.
2.4 Turbidometric assay
This was carried out by inoculating 1% pectin broth medium with fungal culture filtrates and observing the turbidity change spectrophotometrically at 450 nm. 50ml of sterile 1% pectin broth taken in 250ml conical flask was inoculated with different species of Aspergillus separately and incubated at room temperature for 7 days. After incubation, the culture filtrate was collected and used for further assay. 8ml of 1% pectin broth was transferred into 5 test tubes. 2ml of culture filtrate of each organism was added into different tubes and labeled accordingly. To the control tube 2ml of distilled water was added. Optical density reading was taken for every 1 hour interval at 450 nm for 5 hours. Graph was plotted by taking optical density (OD) on X-axis and time interval on Y-axis.
2.5 Assay of polygalacturonase
This was carried out using culture filtrate of A. flavus, A. niger, A. ochraceous and Aspergillus sp., 1% of pectin solution in O.2M citrate phosphate buffer, 40% potassium sodium tartrate, DNS reagent prepared by dissolving 1g of dinitrosalicylic acid, 200mg crystalline phenol and sodium sulphite in 100ml of 1% NaOH solution, glucose, distilled water [10]. The concentration of reducing sugar was estimated using dinitrosalicylic acid (DNS) method. The amount of reducing sugars present in the sample was calculated using the standard graph, obtained using standard glucose solution, following the same procedure. One unit of enzymatic activity (U) was defined as 1μ mol of reducing sugars released per minute [10].
3. Results and Discussion
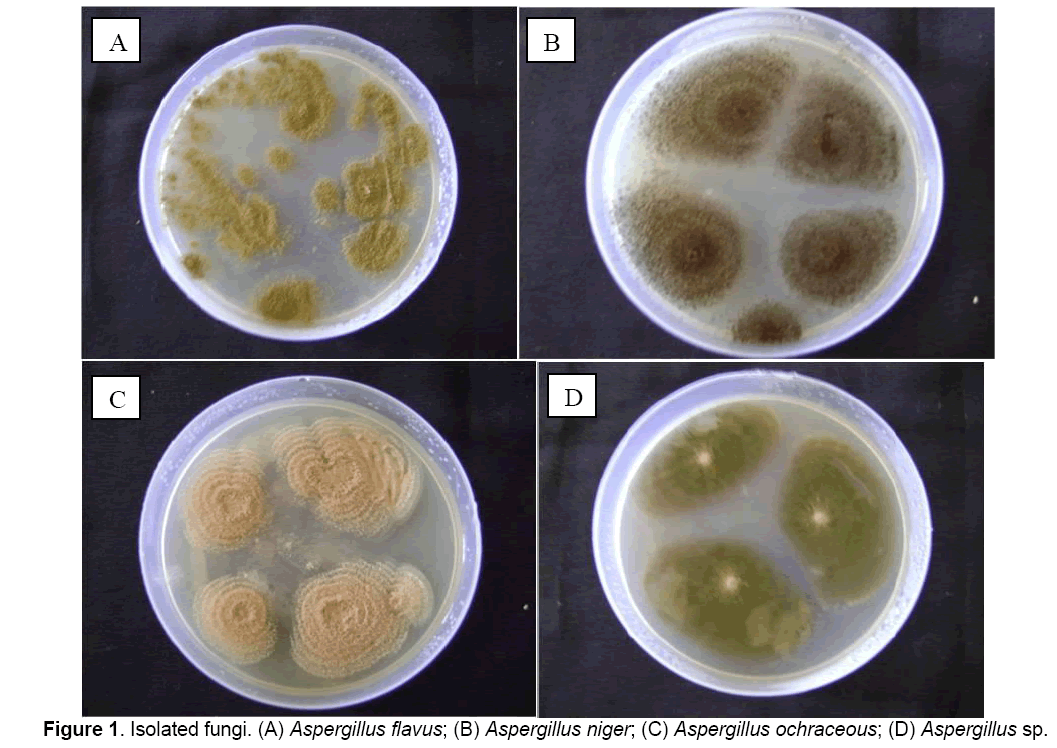
3.1 Isolation of fungi
Following fungi were isolated and identified based on cultural characteristics and sporulation: A. flavus, A. niger, A. ochraceous, and Aspergillus sp., (Figure 1).
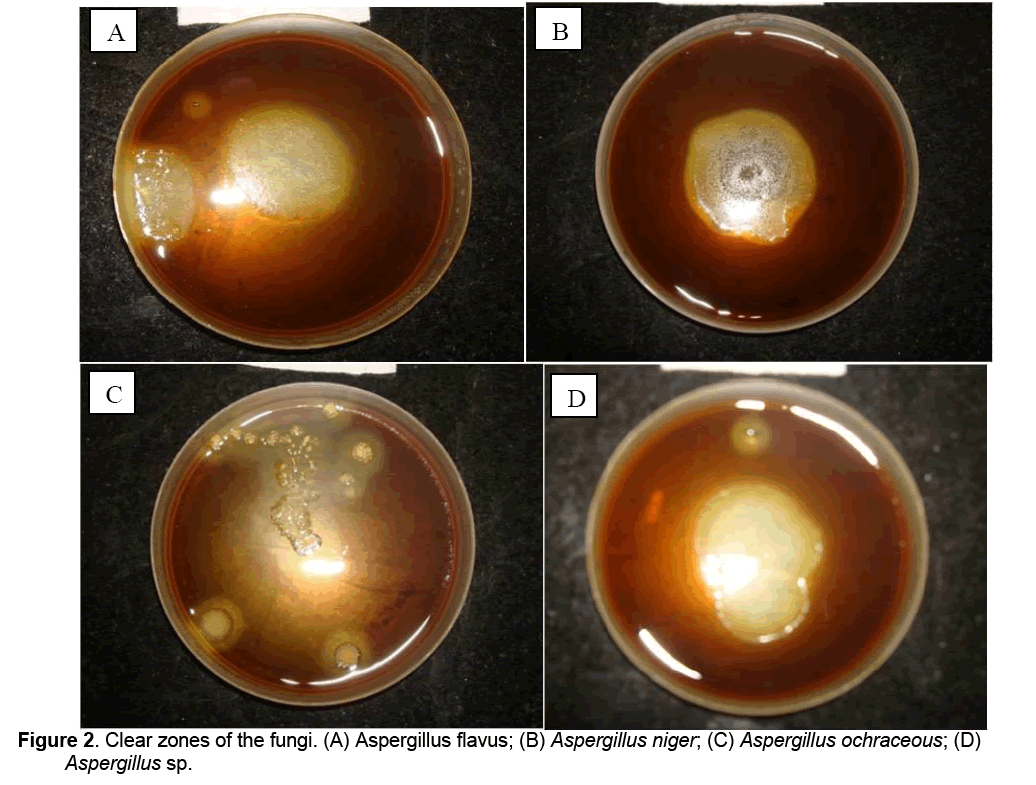
3.2. Plate Assay of depolymerized pectin
After 72 h of incubation, cultures of all the tested fungi viz., A. flavus, A. niger, A. ochraceous, Aspergillus sp., on pectin agar plates showed clear zones on treatment with iodine potassium iodide solution indicating the pectinolytic ability of these fungi (Figure 2).
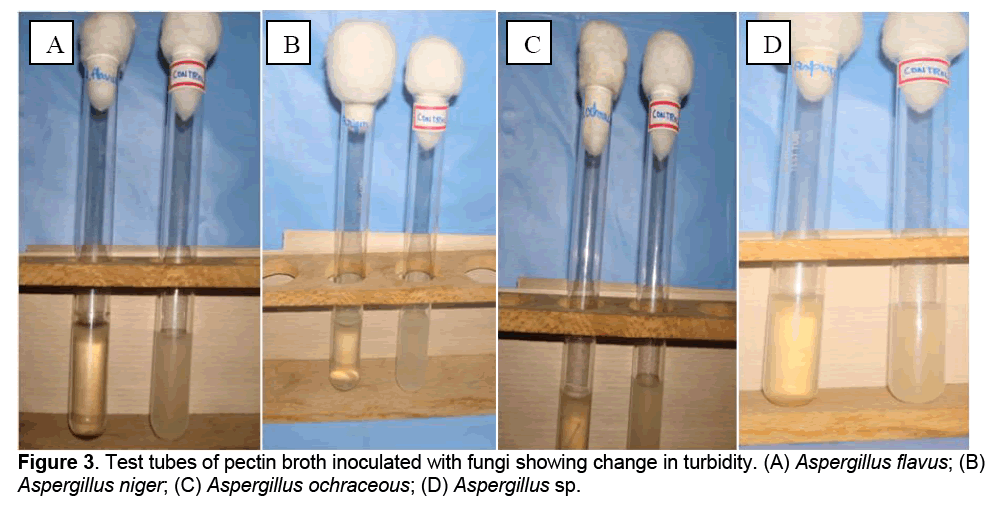
3.3 Turbidity Assay
After 5 days of incubation, change in turbidity was observed in pectin broth tubes inoculated with A. flavus, A. niger, A. ochraceous and Aspergillus sp., as against control (Figure 3).
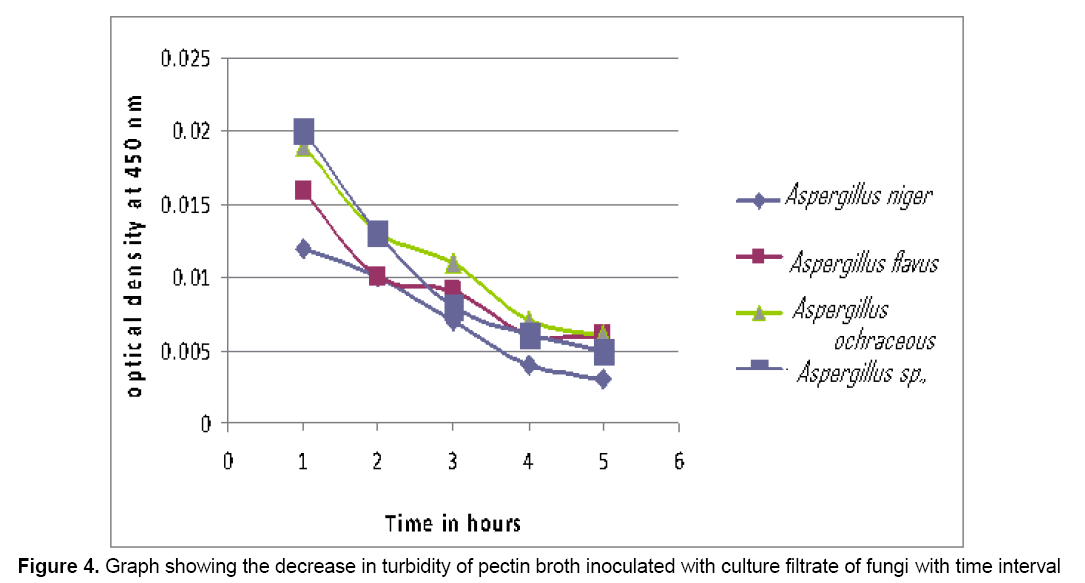
3.4 Turbidometric Assay
The OD values decreased with time interval indicating the presence of pectin degrading enzymes in culture filtrate of all the tested fungi (Figure 4).
3.5. Assay of Polygalacturonic acid
Good amount of reducing sugars were observed in the pectin solution after treatment with culture filtrates of all the fungi (Table 1) which indicate the ability of all fungi (A. niger, A. flavus, A. ochraceous and Aspergillus sp.) to produce polygalacturonase as mentioned in Table 2.
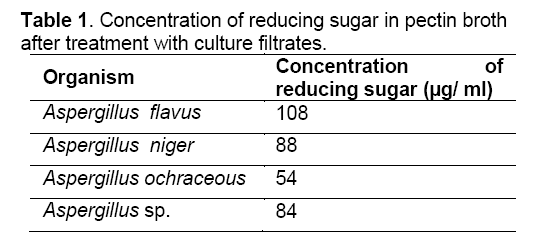
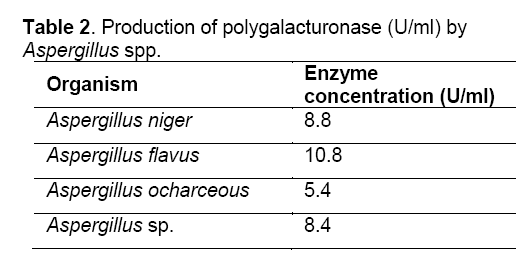
Saphrophytic fungi are known to be good producers of pectinolytic enzymes. In the present study, the fungi studied viz., A. flavus, A. niger, A. ocharceous and Aspergillus sp., were isolated on pectin agar medium. The pectinases are inducible by the polymers they degrade [11]. All the fungi studied produced pectinolytic enzymes as evident from the results obtained in plate assay, turbidity assay, turbidometric assay and polygalaacturonase assay. These assays have been used for the screening of the pectinolytic activity of fungi [10,12]. Good amount of reducing sugar were observed in the pectin solution after treatment with culture filtrates of all the fungi indicating their ability to produce polygalacturonase enzyme. Similar results were reported by other workers with Aspergillus fumigatus, Neurospora crassa, and Rhizopus nigricans [13-15]. The pectinolytic activity was found in culture filtrate and it is reported that crude solutions having specific properties could be more advantageous than purified pectinase preparations [10]. Better utilization of these isolated fungi requires further studies.
References
- Keppler, F., Hamilton, J.T., Brass, M., Röckmann, T., (2006) Methane emissions from terrestrial plants under aerobic conditions. Nature, 439: 187–191.
- Arunachalam, C., Asha, S., (2010) Pectinolytic enzyme - A review of new studies. Adv Biotech J Online, https://www.advancedbiotech.in/online
- Wikepedia., (2013) Pectinase. (Cited on 12.03.2013), Available from: https://en.wikipedia.org/wiki/Pectinase
- Semenova, M., Sinitsyna, O., Morozova, V., et al., (2006) Use of a preparation from fungal pectin lyase in the food industry. Appl Biochem Microbiol, 42: 598-602.
- Jayani, R.S., Saxena, S., Gupta, R., (2005) Microbial pectinolytic enzymes: A review. Process Biochem, 40: 2931-2944.
- Lang, C., H.Dornenburg. 2000. Perspectives in the biological function and the technologicalapplication of polygalacturonases. Appl Microbiol Biotechnol, 53: 366-375.
- Gummadi, S.N., Panda T., (2003) Purification and biochemical properties of microbial pectinases – a review. Process Biochem, 38: 987-96.
- Hoondal, G.S., Tiwari, R.P., Tewari R., et al., (2002) Microbial alkaline pectinases and their industrial applications: a review. Appl Microbiol Biotechnol, 59: 409-418.
- Gysler, C., Harmsen, J.A., Kester, H.C., et al., (1990) Isolation and structure of the pectin lyase D-encoding gene from Aspergillus niger. Gene, 89: 101-108.
- Soares, M.N.C., da silva, R., Gomes., (1999) Screening of bacterial strains for pectinolytic activity. Characterization of polygalacturonase produced by Bacillus sp. Rev Microbiol, 30: 299-303.
- Hadj-Taieb, N., Ayadi, M., Khlif, M., et al., (2006). Fermentor production of pectinases on gruel, a local byproduct and their use in olive oil extraction. Enzyme Microb Technol, 39: 1072-1076.
- Salamao, T.M.F., Amorim, A.C.R., Alves, V.M.C., et al., (1996) Isolation of pectinase hyperproducing mutants Pencillium expansum. Rev Microbiol, 27: 15-18.
- Phutela, U., Dhuna, V., Sandhu, S., et al., (2005) Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Braz J Microbiol, 36: 63-69.
- Polizeli, M.L., Jorge, J.A., Terenzi, H.F., (1991) Pectinase production by Neurospora crassa: purification and biochemical characterization of extracellular polygalacturonase activity. J Gen Microbiol, 137: 1815-1823.
- Ros, J.M., Saura, D., Salmeron, M.C., et al., (1993) Production of pectic enzymes from Rhizopus nigricans cultures with different sources of carbon. Ann Microbiol Enzymol, 43: 71-76..

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences