In vitro Propagation of a Tuberose Plant (Polianthes tuberosa L.)
Sangavai C., Chellapandi P.
1Department of Biotechnology,Dhanalakshmi Srinivasan College of Arts and Science for Women,Perambalure-621212,Tamilnadu,India
2Department of Bioinformatics,School of Life Sciences,Bharathidasan University,Tiruchirappalli- 620024,Tamilnadu,India
Abstract
The tuberose plants are gaining an importance in pharmaceutical and perfume industries because of their peculiar secondary metabolic reactions for the synthesis of various commercial valuable compounds. Although tuberose plants are having a limitation to grow in nursery in worm countries, like India, so that a tremendous application of plant tissue culture has been used for in vitro cultivation of such plants. In this present work, the influence of growth regulators on in vitro shoot formation and regeneration was studied in a tuberose plant Polianthes tuberosa L. Rhizome was used as explant to culture on solid Murashige and Skoog (MS) medium containing different concentrations of indol-3-acetic acid (IAA) and/or 6- benzylaminopurine (BAP). The presence of BAP (1.5mg/l) was showed higher explant regeneration and shoot differentiation frequency (2.2±1.2 shoots/explant), producing about three fold as compared to control. After regenerating this plant through tissue culture, the leaves of the same plant was used for plant chemicals analysis qualitatively, suggested the some of the metabolites were enhanced with more extent. Thus, this work presents an appropriate proportion of BAP and IAA will, perhaps, be helpful for regeneration and cultivation of this plant in small scale nursery.
Keywords
Tissue culture; Tuberose plant; Polianthes tuberosa; Plant hormones; Perfumes; Shoot induction.
1. Introduction
Plant researches have been increased all over the world and large body at evidence have been collected to show immense potential of medicinal plants used in various traditional systems [1-2]. Plants have long been used in perfumery as a source of essential oils and aroma compounds. These aromatics are usually secondary metabolites produced by plants as protection against herbivores,infections,as well as to attract pollinators. Plants are by far the largest source of fragrant compounds used in perfumery [3].
Plant tissue culture is a practice used to propagate plants under sterile conditions,often to produce clones of a plant,which relies on the fact that many plant cells have the ability to regenerate a whole plant (totipotency). Single cells,plant cells without cell walls (protoplasts),pieces of leaves,roots,or rhizomes can often be used to generate a new plant on culture media given the required nutrients and plant hormones. In tissue-culturing of plant cells,plant growth regulators are used to produce callus growth,multiplication,and rooting [4- 9]. Among different plant growth hormones so far studied,IAA and BAP are considered to be more important for in vitro propagation of plants through tissue culture techniques [9-10]. Plant cells synthesize IAA from tryptophan. It has many different effects,as all auxins do,such as inducing cell elongation and cell division with all subsequent results for plant growth and development. A recent application of plant tissue culture is used for the production of economically valuable chemicals [11- 12]. The main chemical compounds are methyl benzoate,methyl anthronilate,benzyl alcohol,butyric acid,eugenol. nerol,farnesol and geraniol [2-3].The tuberose is a night-blooming plant thought to be native to Mexico along with every other species of Polianthes. The tuberose grows in elongated spikes up to 45cm long that produce clusters of fragrant waxy white flowers that bloom from the bottom towards the top of the spike [6,8]. It has long,bright green leaves clustered at the base of the plant and smaller,clasping leaves along the stem. It thrives in sunny spots and bloom in late summer,and its tall stems and rather sparse,grass-like foliage make them ideal for inter-planting [13]. Tuberosa is a popular flower in floras arrangements and their scent is used to produce perfumes the world over. Though different flowering plants have been exploited to produce perfumes,a research and application of tuberose plant,Polianthes tuberosa have been limited in India. In Tamil Nadu it is called as Sambangi or nilasambangi and traditionally used in all type of garlanding especially in south Indian marriages.
Thus,this work was aimed to propagate the tuberose plant,P. tuberosa L. under in vitro condition by using different combination and concentrations of IAA and BAP in MS medium. The regenerated plant was also tried to analyse the presence and absence of some plant chemicals. As tissue culture has valuable meaning this present effort would be helpful in many ways for researchers and farmers.
2. Methods
2.1 Explant collection and sterilization
Pre-cooled rhizome (2°C for 6 weeks) of P. tuberosa procured from Transgenic Institute of Biotechnology and Bioinformatics Research,Tiruchirappalli,India. Young rhizome explants after removing the leaves were washed thoroughly under running tap water for half an hour to remove surface adhering contaminants. The explants were then treated with detergents solution for 5min followed by surfactant treatment for 10min. Then the alcohols wash for just washing off the contaminants followed by 0.1% mercuric chloride treatment for 2 to 3min. The material then thoroughly washed 2-3 times with sterile distilled water [14].
2.2 In vitro propagation
The explants were placed on solid Murashige and Skoog's (MS) basal medium [15] containing 3% (w/v) sucrose,0.8% (w/v) Difco agar,and various concentrations of BAP (6-benzylaminopurine) and IAA (indol-3-acetic acid) in combination used for callus and shoot proliferation. These media were supplemented with growth regulators as indicated in the text and adjusted to pH 5.8 prior to autoclaving at 120oC for 20min. Culture media (25ml) were dispensed into 50 ml test tubes and sealed with parafilm. These were maintained under long day condition (16hrs light at 360 nmol·m-2·s-1 3500 Ingelec fluorescent tube,27°C day/ 21°C night) [14]. All experiments were replicated four times. Calli were visible within 2 weeks along the cut edges. Callus tissues were removed from the explants and sub-cultured 3-week intervals onto the same media upon which they were initiated. The effect of hormones on shoot proliferation was studied and effort was made determine the appropriate hormone combinations for optimal shoot proliferation. The statistical analysis based on mean values per treatment was made using the technique of analysis of variance. The comparative LSD multiple range test (P = 0.05) was used to determine differences between treatments.
2.3 Phytochemical analysis
The plant was dried at room temperature and made into powder further analyses. The ethanol was used as a solvent to extract the plant through Soxhlet apparatus. This coarse powder was extracted with acetone by continuous percolation for 24hrs at 55- 60°C and then extract was filtered and the solvent was removed by distillation under reduced pressure. The secondary metabolites of plant were qualitatively tested according to the standard methods [16].
3. Results
The plant growth regulators are widely used for callus and shoot induction in tissue culture studies. Therefore,we studied the effect of growth hormones,particularly IAA and BAP on callus and shoot induction of a tuberose plant,P. tuberosa in laboratory condition. To induce the calli from explant (rhizome) different concentration of IAA (0.5-3.5mg/l) and 0.5mg/l of BAP used and the results are presented in Table 1.

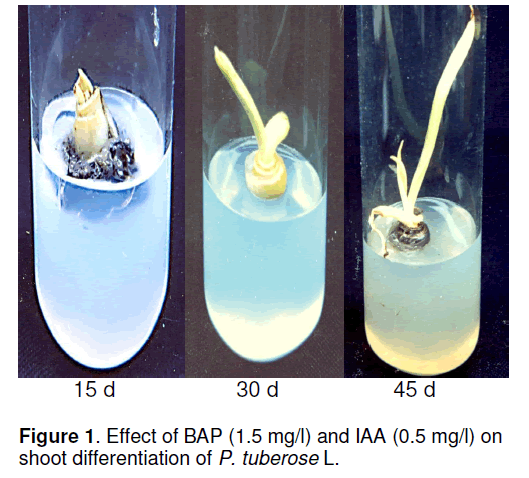
This revealed that the frequency of callus produced/explant was raised with increasing concentration of IAA up to 3.5mg/l. The rate of callus induction frequency was gradually raised and reached maximum to 37.8±1.2 at 3.0mg/l concentration. These calli were sub-cultured on MS medium containing sucrose (3%) and different concentrations of BAP (0.5-2.0mg/l) and 0.5mg/l IAA for shoot differentiation. The results of the concentrations of BAP effects on shoot induction are shown in Table 2 and the shoot growth is depicted in Figure 1.

As similar to callus growth,frequency of shoot/explant was raised with increasing concentrations of BAP,but the shoot growth was retarded at 2.0mg/l concentration. The best shoot growth (2.2±1.2) was found when we used 1.5mg/l of BAP used as a plant growth hormone. This result was comparatively better than the growth of a control. After regenerating this plant using MSsucrose medium (3%) with BAP and IAA,the plant secondary metabolites were qualitatively tested. Among various phytochemicals screened from this plant,steroids,triterpenoids,alkaloids,tannin,sapanins,xanthan protein,flavonoids and carbohydrates were found where as fatty acids and phenolic compounds were absent in the acetone extract of this plant leaves.
4. Discussion
In the absence of NAA (naphthalene acetic acid),only a few cultures (20%) are established from the basal portion of the explant,which indicating the endogenous hormones required for morphogenetic responses are already present in the explant. Urea and thiourea derivatives,the most important group of non-purine cytokinins are widely distributed in plants and have an important regulatory role in plant growth and development [5,12]. Different urea derivatives are applied in vitro and ex vitro to enhance the growth and mass production in oriental hybrid lily and other bulbous crops [11]. Yonova and Guleva also reported that nonpurine cytokinin derivatives of urea and thiourea play important regulatory role in plant growth and development [5]. Hutchinson et al evaluated the potential of thidiazuron (TDZ),a phenyl urea,BAP,a cytokinin and NAA,an auxin,on in vitro propagation of tuberose (P. tuberosa L.) from shoot tip explants [6]. In this study,the shoot was initiated in the cut end of the explants after 15d,and the highest frequency of shoot induction (2.2) was observed in MS medium containing 1.5mg/l BAP and 0.5mg/l IAA. When the same shoot was sub-cultured,in 50- 55 d a considerable improvement of shoot was observed in MS medium enriched with 1.5 mg/l BAP and 0.5 mg/l IAA. TDZ,at low concentrations,was more potent than BAP in increasing shoot length and quality as well as the number of leaves per shoot of P. tuberosa L. [6]. Both concentrations of NAA in combination with BA did not affect the regeneration response and number of bulblets per explant of oriental lily [7]. However,this work reported that shoot growth was more significant on MS medium in the presence of 3.0mg/l IAA and 0.5mg/l BAP using rhizome explant. The highest frequency of callus induction (37.8±1.2) was observed on MS medium containing 3.0mg/l IAA and 0.5mg/l BAP. 90% of the shoot produced at 45 d when the shoot transferred to a regeneration medium supplemented with 2.5mg/l BAP and 1.0mg/l IAA. In addition,the highest numbers of shoot/explant were obtained with initial pH values between 5.5 and 6.0. Similarly,shoot differentiation occurred when light-green compact callus of Hybanthus enneaspermus L. Muell was transferred to MS medium supplemented with 8.8 μm BA and 2.6μm NAA; the highest percentage of calli forming shoots (66.6±4.8%) and the highest numbers of shoots (8.9±0.3) have achieved in this medium [10]. Differentiated shoot buds of H. enneaspermus L. Muell elongated to 4–5cm within 4 weeks. This response could be improved in when glucose was used as a carbon source for a larger flowered purslane [4],and using more concentrations of casein hydrolysate (500mg/l) and potassium phosphate (1.86mm) for H. enneaspermus L. Muell [10]. Similarly,in this study a considerable growth of shoot proliferation and regeneration observed in a 45 d culture.
Cytokinin induced multiple shoot formation of P. tuberosa cultivars Shringar (single type) and Suvasini (double type),however,the addition of auxin increased the regeneration response. A multiple shoot induction of 100% was obtained with 2mg BAP+0.1mg IAA/l. Shoot length increased with the increase in BAP level. This present work was also gave the similar results,but the concentrations of BAP (1.5mg/l) and IAA (0.5mg/l) was slightly changed.
A rooting percentage of 100%,along with the highest average number of roots (16.70 in P. tuberosa L. Shringar and 15.8 in P. tuberosa L. Suvasini),was recorded for 0.2mg IAA + 0.25mg IBA/l. The longest root (1.39cm in Shringar and 1.33cm in Suvasini) was obtained with 0.5 mg IBA/litre [9]. Rooting was achieved on the shoots of H. enneaspermus L. Muell using half-strength MS medium containing 4.8μm IBA [10]. No additional treatment is required for rooting from a larger flowered purslane,which occurred in all regenerated shoots,with a 100% survival rate of plants in the glasshouse [4]. Thus,root proliferation in this plant can be brought out according to these ways.
High free putrescine and low spermine and spermidine levels in P. tuberosa L. were associated during initial stages of dormancy [12]. A gradual increase of bound ABA (abscisic acid) was observed during release of rest periods of P. tuberosa L. Acidic and bound phenols vary with the dormant phases. The levels of IAA remained low during the early stages of bulb sprouting in P. tuberosa L. but increased rapidly thereafter [13]. The dramatic reduction in the free ABA content in corm tissues are correlated with floral initiation and flower development in P. tuberosa [8]. Apart from the plant growth regulators,nitrogenous compounds might also increase the protein level in the explant of lily during differentiation resulting in higher number of bulblets [7]. According to earlier investigations,we revealed that a specific secondary metabolite biosynthesis and accumulation in a plant is depending on the hormones involving in plant growth differentiations at different developmental stages. Therefore,incorporating specific plant growth regulators like IAA and BAP in a growth medium (MS) can support the synthesis of chemical compounds of the interests,especially compounds related to perfumes and aroma in P. tuberosa L.
5. Conclusions
Overall,we conclude that IAA and BAP at a specific concentration in MS medium are needed for the effective proliferation of callus and shoot of this plant from rhizome explant. One of the main challenges facing production and marketing of good quality tuberose cut flowers is the lack of clean planting material as the resource (Hutchinson et al.,2004). Another one noteworthy of this present work is that as India like worm countries we could not propagate P. tuberosa widely so that our preliminary attempt will perhaps be helpful for looking further research work and commercialization. Moreover,this effort will make more advantages for in vitro cultivation of P. tuberose L. for screening secondary metabolites at a laboratory-scale and also provide an insight to farmers for plantations in glasshouse.
Acknowledgements
The co-author is thankful to Principal,Dhanalakshmi Srinivasan College of Arts and Science for Women,Perambalore,India for permission to carry out this work.
References
- Dhanukar,S.A.,Kulkarni,R.A.,Rege,N.N. (2000) Pharmocology of Medicinal plants and natural products. Indian J Pharmacol,32: S81-S118.
- Kaufman,P.B. (1999) Natural products from plants. (1999),CRC Press.
- Edwards.,Michael. (2006) Fragrances of the world. Crescent House Publishing.
- Badr-Din,R.H.,Jean-Pierre,Z. (1995) In vitro culture and plant regeneration of larger flowered purslane. Plant Cell Tissue Organ Culture,41: 281-283.
- Yonova,P.,Guleva,E. (1997) Plant growth regulating activity of some novel 1,1'-polymethylene bis (3- arylsubstituted)-thioureas. Bulgarian J Plant Physiol,23: 72-79.
- Hutchinson,M.J.,Onamu,R.,Obukosia,S. (2004) Effect of thidiazuron,benzylaminopurine and naphthalene acetic acid on in vitro propagation of tuberose (Polianthes tuberosa L.) from shoot tip explants. J Agri Sci Technol,6(1): 48-59.
- Kumar,S.,Awasthi,V.,Kanwar,J.K. (2007) Influence of growth regulators and nitrogenous compounds on in vitro bulblet formation and growth in oriental lily. Hort Sci (Prague),34(2): 77-83.
- Wei-Ren,S.,Kuang-Liang,H.,Rong-Show,S.,et al. (2002) Abscisic acid affects floral initiation in Polianthes tuberosa. J Plant Physiol,159(5): 557-559.
- Krishnamurthy,K.B.,Mythili,J.B.,Meenakshi Srinivas (2001) Micropropagation studies in "single" vs. "double" types of tuberose (Polianthes tuberosa L.). J Appl Hort,3(2): 82-84.
- Prakash,E.,Sha Valli Khan,P.S.,Sairam Reddy,P.,et al. (1999) Regeneration of plants from seed-derived callus of Hybanthus enneaspermus L. Muell.,a rare ethnobotanical herb. J Plant Cell Reports,18(10): 873- 878.
- Nhut,T.,Van,B.,Silva,J. (2002) Changes of shoot regeneration potential of oriental hybrid lily. Adv Horti Sci,9: 79–82.
- Shweta,S.,Nagar,P.K. (2005) Alterations in endogenous polyamines in bulbs of tuberose (Polianthes tuberosa L.) during dormancy. Scientia Hort,105(4): 483-490.
- Nagar,P.K. (1995) Changes in abscisic acid,phenols and indoleacetic acid in bulbs of tuberose (Polianthes tuberosa L.) during dormancy and sprouting. Scientia Hort,63: 77-82.
- Lindsey,K. (1997) Plant tissue culture manual. (1st Edn.),Kluwer Academic Publishing.
- Murashige,T.,Skoog,F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant,15: 473-497.
- Harborne,A.J. (1998) Phytochemical methods (3rd edn.). Springer.
- Yonova,P.A.,Izvorska,N.D.,Lilov,D.T,et al. (1989) Action of the synthetic cytokinins of the urea type on the growth and development of cytokinin-dependent tissue cultures. Comptes Rendus de l’Academie Bulgare des Sci,42: 135–138.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences