Hepatogenic Differentiation of Rabbit BM-MSC using Non-coated Flask
Ola G. Wasel, Adel F. Badria, Abdelaziz A. Mohamed, Mona K. Marei
1Tissue Engineering Laboratories,Faculty of Dentistry,Alexandria University.Champolion Street,Azarita. Alexandria,Egypt.
2Medical Technology Center,71Amanwel St.Smouha,Alexandria,Egypt
- Corresponding Author:
- Adel F. Badria
Tel: +20-011 118 15 286
Fax: +203 4266214
E-mail: afbmecca@yahoo.com
Abstract
The objective of the current work is to develop an invasive culture reproducible media that supports robsut differentiation of bone marrow mesenchymal stem cell to functionsl hepatocyte-like cells from Newzeland rabbit models. We believe this is the first report utilizing rabbit model for mesenchymal stem cells from extrahepatic proportions. In this article we were able to demonstrate characterization of rabbit hepatocyte-like cells at morphological, molecular and functional level using non-coated flask
https://blogum.blogaaja.fi/
https://blogum-1.jimdosite.com/
https://blogummm.edublogs.org/
https://blogummm.websites.co.in/
https://blogum18.wordpress.com/
https://benim-blogum.jigsy.com/
https://fuiegs-symbeaurds-build.yolasite.com/
https://blogum-03.webselfsite.net/
https://blogummm.mystrikingly.com/
https://blogum.splashthat.com/
https://blogum3.webnode.com.tr/
https://blogum.odoo.com/
https://blogum.creatorlink.net/
https://whiteseotr1-s-site.thinkific.com/enrollments
https://blogum.estranky.cz/
https://653ba4fbb538c.site123.me/
https://blogum12m.blogspot.com/
https://blogum.hashnode.dev/
https://whiteseoturkey1.wixsite.com/blogum
https://sites.google.com/view/blogummm/
https://codepen.io/blogum
https://blogumm.livejournal.com/
https://wakelet.com/@blogum82816
https://www.homify.com/users/9538383/blogum
https://lessons.drawspace.com/profile/323613/blogum
https://my.desktopnexus.com/blogum/
https://writeupcafe.com/profile/BLOGUM/
https://www.pearltrees.com/blogum
https://www.easyfie.com/blogum
https://pharmahub.org/members/27615/profile
https://www.zupyak.com/u/blogum/posts
https://www.metroflog.co/blogum
https://www.fuzia.com/fz/blogum-blogum
https://tr.pinterest.com/blogum12/
https://my.getjealous.com/blogum
https://micro.blog/blogum
https://www.tumblr.com/blogummm
https://hub.docker.com/u/blogum
https://fire.blogfree.net/?act=Profile&MID=1342323
https://blogum.pixnet.net/blog
https://www.threadless.com/@blogum/activity
https://blogum.neocities.org/
https://blogum12.amebaownd.com/
https://teletype.in/@blogum
https://ubl.xml.org/users/blogum
https://educatorpages.com/site/blogum/
https://blogum.onlc.fr/
Keywords
Rabbit; BM-MSC,hepatogenic-like cell; non-coated Flask.
1. Introduction
Egypt has the largest epidemic C virus in the world. The recently released Egyptian Demographic Health Survey (EDHS) tested a represented sample of the entire country for the HCV antibody revealed that the overall prevalence (percentage of people) positive for antibody to HCV was 14.7%. While the survey showed that the new infections rate increased over 6/1000 each years,with almost 500,000 individuals getting infected,70,000 of them are children [1].Current treatment for the chronic hepatitis C is expensive,is often accompanied by burdensome side effects and sadly [2],fails in almost half of cases. The ability to predict such failures prior to treatment could save a great deal of pain and expenses for patient with HCV e.g. through genetic markers,yet although these methods could be promising but required great deal of validation [3,4]. While there is no regular cure for hepatitis and no completely effective treatment,the high prevalence of chronic liver diseases in Egypt has led to increasing numbers of patients suffering from end-stage liver disease. Liver transplantation (LT) is the most effective treatment for the end-stage liver disease and selective primary hepatic malignancies. The life-expectancy of a liver transplant recipient depends on the cause of the liver cirrhosis or liver failure. Although the survival rate for non-viral cases could reach up to 95% for one year,80% for 5 years and 70% for 10 years,yet for viral disease it is far less due to recurrence of viral disease that damages the transplanted liver. [5] Although liver transplantation become accepted as health care standards,the ethical,legal equity and considerable risks for living donors transplant,all issues need to highlighted in Egypt in addition to donor shortage,need for a life-longed immunosuppressive therapy,high cost can't be available to all people [6,7].The transplantation a hepatocyte mass equivalent to 10% of patients' liver would be sufficient to normalize the metabolic situation in many enzyme deficiencies,Cell transplantation is more desirable with less invasive technique that associated with lower mortality and morbidity and can be repeated in case of recurrence [8]. However,one major limitation of cell-based therapies for liver disease is the availability of human hepatocytes,which makes a wider use of these techniques,will not be possible until adequate numbers of functional cells for transplantation become more readily available [9].
There were numerous investigations in past 10 years about generation of hepatocyte-like cells from different types of extrahepatic stem or precursor cells that open new exciting opportunities for cell therapy [10]. These include multipotent stem,progenitor-like cells that have been isolated from tissues outside the liver e.g Bone marrow,Umbilical cord,Amniotic fluid,and adipose tissues [11,12]. Preliminary data suggest that mesenchymal stem cells have properties that all for transplantation across major Histocompatibility complex barriers,They have the ability to home to damaged tissue,Because mesenchymal stem cells (MSCs) have immunomodulatory effects against alloantigens,they may be used to treat rejections of organ allografts such as liver,kidney,heart and intestinal transplant. [13-19]The objective of the current work is to develop an invasive culture reproducible media that supports robsut differentiation of bone marrow mesenchymal stem cell to functionsl hepatocyte-like cells from Newzeland rabbit models.
We believe this is the first report utilizing rabbit model for mesenchymal stem cells from extrahepatic proportions. In this article we were able to demonstrate characterization of rabbit hepatocyte-like cells at morphological,molecular and functional level using non-coated flask.
2. Microflora and diseases of neem
Isolation of mesenchumal stem cells from Rabbit bone marrow samples
Bone Marrow aspirates (2 ml) were obtained from iliac crest of 3-4 months old V-line white rabbits weighted 3-4 Kg under local anesthesia. Marrow media (Dulbecco’s modified Eagle media supplemented with 10% fetal bovine serum (FBS),1% L-glutamine,1% Pencillin Streptomycin and 2 % HEPES buffer,all from Lonza,Belgium) were added to each sample and centrifuge at 453 g (Cooling centrifuge with swing bucket rotor Model # 5810R) for 7 minutes.
The pellet was washed twice,cells counting and viability were achieved,and then the cells inoculated by density 12 x 105 cells/Cm2 in T-25 Cm2 flasks. The Flasks were incubated at 37o C with 5% CO2 and 95% humidity (Revco,Model # RC 05000TWBB,Reveco- USA). After 4 days,the media were discarded,the flasks were washed twice by PBS and fresh media added and all flasks incubated until cells reached 80-90% confluence.
Once cells reached confluency (day 20); cells were trypsinized using Trypsin/EDTA (200mg/L versene EDTA,170000 U trypsin,Lonza,Belgium). Viability test was performed using trypan blue and cell morphology was assessed via inverted phase contrast microscope (TE2000;Nikon,Tokyo,Japan) and The DXM1200F digital camera and software ACT-1 for the DXM1200F (version 2.20;Nikon). Cells were inoculated by density 2 X 103 cells/Cm2 T-25 Cm2 flasks until 30 days [20-23].
Characterization of Mesenchymals stem cells: Colony forming unit-fibroblast assay (CFU-f)
Mesenchymal stem cells that were cultured for 14 days then 10 x 106 were cultured to obtain two concentrations 5X105 cells/well and 1X106 cells/well for duplicate assays in six-well plates (NuncTM,Denmark),the cells were plated into well - with mixing to provide homogenous distribution of cells inside the wells- for 10 days at 37C in 5% CO2. At the 10 days,the media were removed and the six-well plates were stained with 90% glacial acetic acid and 5% of 0.01% crystal violet (all from park scientific limited UK).
The cells were washed with PBS,fixed with 4% paraformaldehyde in PBS for 25 minutes and wash twice with distilled water,then 0.1% methelyene blue was added to the wells and incubated for 2 hours.
The plates were washed twice with distilled water and the number of colonies was counted. Colonies less than 2 mm in diameter and faintly stained colonies were ignored [24].
Immunocytochemistry Staining of RBMMSCs
RBMMSCs were placed on positively charged slides and incubated overnight in the CO2 incubator at 5% CO2,37 ˚C and 95% humidity. Then slides were rinsed twice in cold phosphate buffered saline (PBS),5 minutes each. The slides were fixed in ice cold methanol for 5 minutes. The samples were rinsed twice with ice cold PBS,5 minutes each.
Cells were incubated with 1% bovine serum albumin (BSA,Fluka) for 30 minutes to block the non-specific antigen sites in the cells to avoid false positive labeling when the primary antibody is applied. Slides were drained without rinsing,and then incubated with mouse anti rabbit monoclonal primary antibody CD146 (Abcam,Cambridge -UK),of diluted concentration of 1: 250 in PBS with 1% BSA. As a negative control,slides were treated similarly,except that the primary antibody was omitted from the solution of incubation.
All the slides were kept in a humidified media overnight at 4°C. After 24 hours the solution was discarded and the slides were washed three times in PBS,5 minutes each.
Rabbit anti Mouse polyclonal IgG rhodamine tagged secondary antibody (Abcam,Cambridge–UK) was applied to the slides in a concentration of 1:250 in PBS with 1% BSA,with high handling precaution as all the next steps were performed in darkness to protect the fluorescence efficiency of the marker from fading. The antibody was incubated for one hour at room temperature. slides were finally washed twice in 5 minutes each. Finally slides were cleared in xylene for 5 min then were mounted and coversliped. slides were then stored in darkness at 4°C ready to e viewed and imaged using confocal scanning laser microscope ≠( 210B0io -Rad,Microscience/Itd-UK) [25].
Mesodermal Differentiation of RBMMSCs
Osteogenic differentiation of RBMMSCs was induced by culturing passage 1 of RBMMSCs in osteogenic medium. When the cells grew to 85% confluence,they were cultured in DMEM containing 10-7 M Dexamethasone (sigma,Belgium),0.05mM ascorbic acid (Sigma Belgium) and 10mM β- glycerophosphate (sigma,Belgium),1%L-glutamine,1% pencillin strepyomycin ,10%FBS and 2% Hepes buffer. Cells were cultured in a humidified atmosphere of 5% CO2 and 95% humidity at 37°C. The medium was changed twice per week. Cells without dexamethasone ascor ic acid and β- glycerophosphate used as negative control.
After 17 days,the cells were exposed to alizarin red staining. The media was removed and the cells were washed with PBS one time. The cells were fixed using 10% Formalin for 30minutes,and then washed with distilled water. 1% Alizarin red solution (pH=4.1) was added to the cells,and left for 10min. The stain was removed carefully and the cells were washed with water three times. Cells then were examined using inverted phase contrast microscope [26].
Hepatogenic differentiation of RBMMSCS:
Cells from passage passage 2 of RBMMSCs at 80-90 % confluence were used in hepatogenic differentiation. Two steps induction of differentiation process was performed as it was described before,yet with some modification the differentiation protocol was performed on non-coated polystyrene flasks by sequential addiation of growth factors,cytokines,glucocorticoids and hormones.
RBMMSCs from 2nd passage were cultured in DMEM containing 10% FBS,1% L-glutamine,1% pencillin strepromycin and 2% HEPES buffer (control medium) for 28 days (the name of the company) were used as negative control.
For hepatogenic differentiation,RBMMSCs were plated 0.5 x 106 cells/Cm2 on an non-coated polystyrene flasks(TPP,the company) and incubated in medium containing control medium supplemented with 20 ng/ml HGF (R&D systems),10 ng/ml bFGF (R&D systems),5mg/L insulin (Sigma,USA),5 mg/l transferrin (Applochem,Germany),5 μg/l selenium (Sigma,USA) and 0.5 μM Dexamethazone (Serva Electrophoresis GmbH,Heidelberg). The cells under hepatogenic differentiation were incubated for 2 weeks,and then proceed for the 2nd step of differentiation. In the second step,containing control medium supplemented with 20ng/ml OSM (Sigma,USA),5mg/L Insulin,5mg/L Transferrin,5μg/L Selenium and 0.5μM Dexamethasone was added to RBMMSCs for another two weeks to achieve cell maturation. The medium were changed twice weekly. Cells were monitored daily via phase contrast inverted microscope while culture media was collected for the albumin and urea production till 28 days [27,30].
Reverse transcription polymerase chain reaction
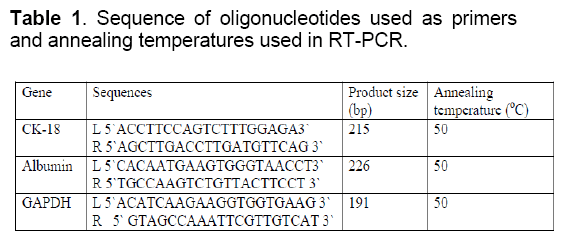
Total RNA was extracted from the cells QIAamp RNA Blood Mini Kit (Qiagen,Germany) according to the manufacturer protocol at day 0,7,14,21 and 28 after induction of hepatognic differentiation. Total RNA isolated from liver tissue served as positive control. The cDNA was synthesized and PCR was performed using RT-PCR gold premix kit (Bioron,Germany). RT-PCRs were conducted using approximately 0.7μg RNA to amplify hepatocyte specific genes listed in Table 1. Glyceraldehyde 3 phosphate dehyderogenase (GAPDH) was used as housekeeping gene. cDNA synthesis reactions were performed as follows: 42°C for 60 minutes then 94 °C for 10 minutes for reverse transcriptase inactivation and PCR initiation. PCR amplification was carried out at 95°C for 45 seconds,at annealing temperature for 45 seconds,and 72°C for 45 seconds for a total of 35 cycles,and final extension at 72°C for 7 minutes using thermocycler (Biometra T- personal). PCR tubes with no RNA template was used as negative control for each primer. Amplified PCR products were separated on a 1.5 % agarose gel electrophoresis and the bands were visualized by ethidium bromide (1mg/ml) using UV transilluminator and photographed. 100 bp BLUE extended DNA ladder was used as marker (bioron,germany) [31].
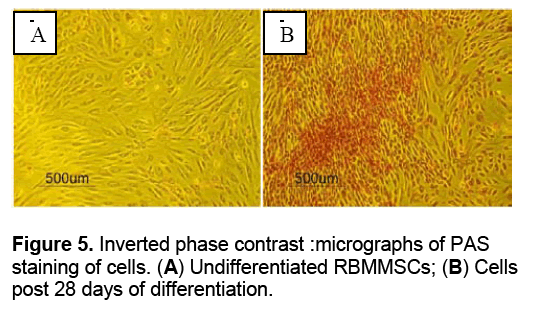
Assessment of functionality of hepatocyte- Like Cells
To assess the functionality of hepatocyte like cells,periodic acid-Schiff's (PAS) staining was performed at day 28 post induction of differentiation to detect intracellular glycogen. Medium was discarded from the flasks and cells were rinsed with PBS three times then fixed with 100 ml/L formaldehyde for 30 minutes. The cells were oxidized in 1% periodic acid for 10 minutes and rinsed thrice in distilled H2O. fterwards cells were treated with chiff’s reagent for 10 minutes rinsed in distilled H2O for 10 minutes and observed under an inverted phase contrast microscope.
Transmission electron microscopy analysis of RBMMSCs
RBMMSCs after 21 days post induction of differentiation were fixed in mixture of 4% paraformaldehyde and 1%glutaraldehyde for 2 hours at 4 °C. Then,they were post fixed with 2% osmium tetraoxide in isotonic buffer at pH=7.4 for 3 hours at 4°C. Following fixation,samples were dehydrated in a graded series of acetone and embedded in epoxy resin for sectioning. Sections of 100 nm were obtained by cutting the samples using ultramicrotome fitted with glass knife. The sections were picked up on copper grids and stained with 1.2 % uranyl acetate for 10-20 minutes then washed with distilled water. 0.4% lead citrate in 10N NaOH was added to the sections for 10-20 minutes then washed with 0.02 N NaOH and finally with distilled water .The grids was examined with Transmission Electron Microscope (Joel jem -100cx,Japan).
3. Results and Discussion
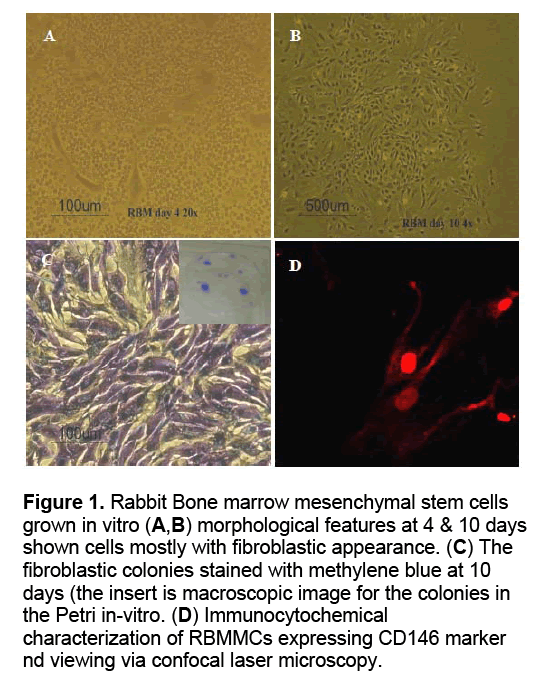
Attached RBMMSCs formed colonies of fibroblastic cells at 4 days,which increased in number and size by 10 days. The number of colonies at 10 days expressed in mean ± SD of 4.5 ± 0.54772 colonies/105 cells and 6.25 ± 2.5 colonies/106 cells,while the colonies size range between 2 mm to 6 mm (Figure 1A-C).
Figure 1: Rabbit Bone marrow mesenchymal stem cells grown in vitro (A,B) morphological features at 4 & 10 days shown cells mostly with fibroblastic appearance. (C) The fibroblastic colonies stained with methylene blue at 10 days (the insert is macroscopic image for the colonies in the Petri in-vitro. (D) Immunocytochemical characterization of RBMMCs expressing CD146 marker nd viewing via confocal laser microscopy.
Immunophenotyping of Immunophenotyping of cultured RBMMSCs revealed their immunoreactivity when treated with marker CD146. The images obtained from the confocal laser microscope have shown the positively stained red fluorescin rhodamine labeled anti-CD146 antibody of bone marrow mesenchymal stem cells,Figure 1D.
Osteogenic induction of RBMMSCs
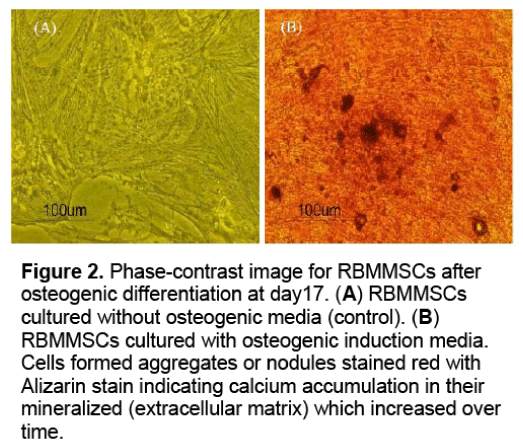
During the induction of osteogenic differentiation,the RBMMSCs became condensed formed multilayers,dense exteracellular matrix (ECM) was observed by day 17 and aggregate into post induction. The mineralized matrix was formed as evidenced by Alizarin red staining (Figure 2 ).
Figure 2: Phase-contrast image for RBMMSCs after osteogenic differentiation at day17. (A) RBMMSCs cultured without osteogenic media (control). (B) RBMMSCs cultured with osteogenic induction media. Cells formed aggregates or nodules stained red with Alizarin stain indicating calcium accumulation in their mineralized (extracellular matrix) which increased over time.
The cells were stained red,indicating the presence of calcium in the ECM. Mineralized matrix was not detected in the control cells which cultured without adding osteogenic differentiation media.
Figure 2 . Phase-contrast image for RBMMSCs after osteogenic differentiation at day17. (A) RBMMSCs cultured without osteogenic media (control). (B) RBMMSCs cultured with osteogenic induction media. Cells formed aggregates or nodules stained red with Alizarin stain indicating calcium accumulation in their mineralized (extracellular matrix) which increased over time.
Hepatogenic differentitation of RBMMSCs into hepatocyte-like cells
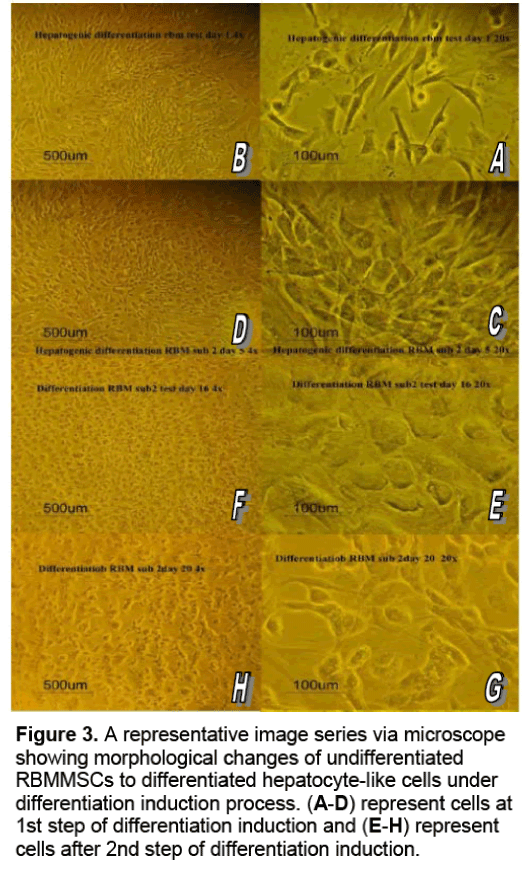
At day 1,cells exhibited a fibroblastic-like morphology (Figure 3 A,B). From one to two days after the incubation,some of fibroblast-like cells start to increase in size and develop a broadened flattened shape with multilateral contours,low nuclear-to-cytoplasmic ratio and some showed round nuclei at the periphery. From the third day the culture were full of polygonal shape cells that form colonies surrounding the flat spindle undifferentiated cells (Figure 3 C,D). Once the 2nd step of differentiation started,almost 85% of the cultured showed the polygonal cells that increased in density and some cells were binucleated at the higher magnification (Figure 3 E,F). At day 16,cells became larger with a broadened,flattened shape and many polygonal shapes,while some binucleated cells appeared with dense,granular-rich cytoplasm (Figure 3 G,H). At day 20,cells showed cord-like arrangement similar to the trabeculae of the liver lobules. With this last described arrangement and morphology by day 20,the cells under hepatogenic induction process revealed marked differences from the cells in the control medium.
Figure 3: A representative image series via microscope showing morphological changes of undifferentiated RBMMSCs to differentiated hepatocyte-like cells under differentiation induction process. (A-D) represent cells at 1st step of differentiation induction and (E-H) represent cells after 2nd step of differentiation induction.
Figure 3 . A representative image series via microscope showing morphological changes of undifferentiated RBMMSCs to differentiated hepatocyte-like cells under differentiation induction process. (A-D) represent cells at 1st step of differentiation induction and (E-H) represent cells after 2nd step of differentiation induction.
Hepatic gene expression via RT-PCR
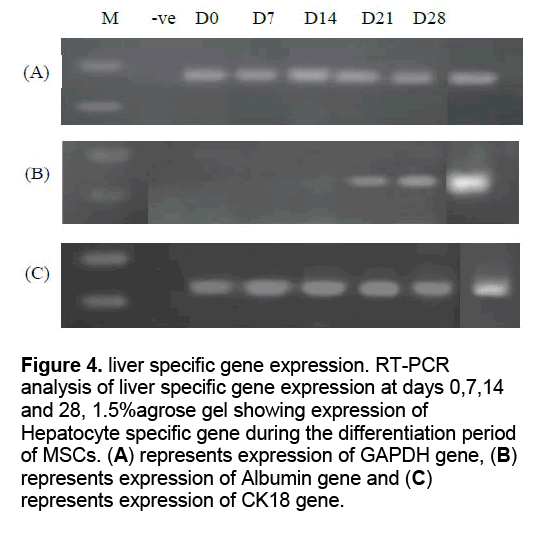
RNA was isolated from cells at days 0,7,14,21 and 28 of differentiation and RT-PCR was done for hepatocyte specific genes,albumin and ck18. Also RT-PCR for GAPDH was performed as housekeeping gene. RNA from liver tissue was isolated and RT-PCR for the same genes was done as positive control. As a negative control for the primers,a no template RT-PCR reaction was run under the same conditions. mRNA expression of GAPDH gene were appeared for all samples with the same intensity indicating that the amount of RNA in all samples was the same. mRNA expression of Albumin ,was firstly appeared at day 21 and continued to day 28 but absent from day 0-14. mRNA expression of Ck18 was appeared at day 0,7,14,21 and 28 of differentiation (Figure 4 ).
Figure 4: liver specific gene expression. RT-PCR analysis of liver specific gene expression at days 0,7,14 and 28, 1.5%agrose gel showing expression of Hepatocyte specific gene during the differentiation period of MSCs. (A) represents expression of GAPDH gene, (B) represents expression of Albumin gene and (C) represents expression of CK18 gene.
Assessment of functionality of hepatocyte-like cells
Glycogen storage was evaluated in MSCs before and after differentiation in order to assess the metabolic capacity of differentiated cells. Undifferentiated cells and differentiated cells after 28 days of differentiation were exposed to PAS staining. The stain was accumulated in differentiated cells only indicating ability of these cells to store glycogen which is one of the main functions of hepatocytes (Figure 5 ).
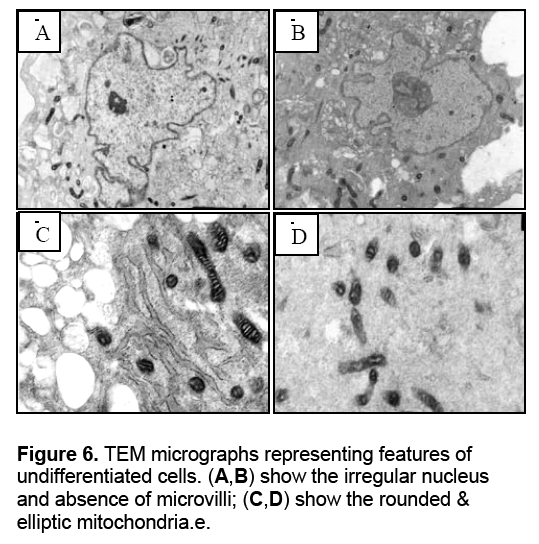
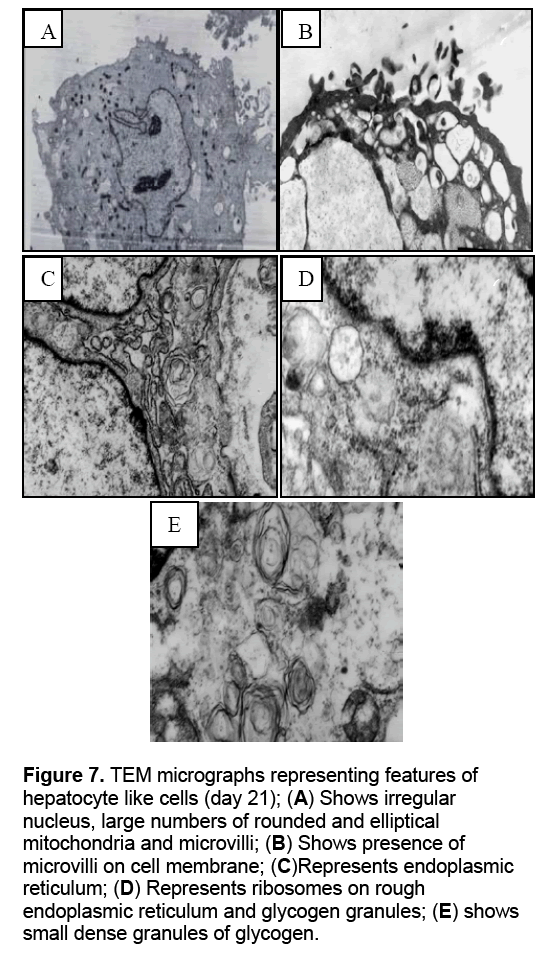
Assessment of functionality of hepatocyte- like cells via transmission electron microscopy
Ultrastructural analysis of RBMMSCs after 21days post induction of hepatogenic differentiation was perforemed using transnmission electron microscope and compared with undifferentiated cells. The ultrastructural studies of hepatocyte-like cells represented that the cells have ultrastructural feature of normal hepatocytes. These features include presence of microvilli and glycogen granules which were absent in undifferentiated cells. Beside these feature,rough endoplasmic reticulum and mitochondria also observed (Figure 6 ,7).
Figure 7: TEM micrographs representing features of hepatocyte like cells (day 21); (A) Shows irregular nucleus, large numbers of rounded and elliptical mitochondria and microvilli; (B) Shows presence of microvilli on cell membrane; (C)Represents endoplasmic reticulum; (D) Represents ribosomes on rough endoplasmic reticulum and glycogen granules; (E) shows small dense granules of glycogen.
4. Conclusion
In this article we were able to demonstrate characterization of differentiation of BM-MSC into rabbit hepatocyte-like cells at morphological,molecular and functional level using non-coated flask.
Acknowledgements
We appreciate the efforts and support of all the researchers in tissue engineering laboratories especially Ramy turkey and Dalia Khamis from animal care unit for their generous aid in aspiration of bone marrow samples and Mahmoud Hamad for aid in SEM imaging.
References
- El-zanaty F,Way A,(2009) Egypt Demographic Health Survey,Cairo,Egypt,Ministry of Health.
- Diaz S,Liu P,Kuhnert wl,Healy M,Princo AM,El-Nageh MM,(2008) Development of the international consortium for blood safety (ICBS) HCV panels,Eastern Mediterranean health journal,14: 427-437.
- OH TS,Rice CM,(2009) predicting response to hepatitis C therapy. J Clin Invest,119(1): 5-7.
- Aurora R,Donlin MJ,Cannon NA,Tavis JE,(2009) Genome-wide hepatitis C virus amino acid convariance networks can predict response to antiviral therapy in humans. J Clin Invest,119: 225-236.
- El-gazzaz GH,Elemi AH,(2010) liver transplantation in Egypt from West to East. Transplantation research and risk management,2: 41-46.
- El-Moghazy WM,Ogura Y,Mutsuko M,Harada K,Koizum A,et al. (2010) Pediatric Living-donor liver transplantation for acute liver failure: Analysis of 57 cases.Transplantat International,23: 828-830.
- Abdeldayem HM,Allam NA,Salah E,Mastafa AA,Kashkorish S,et al.,(2009 Moral and ethical issues in living-donor liver transplant in Egypt. Exp Clin Transplant,7: 18-24.
- Starzl TE,Fung JJ.,(2010) Themes of liver transplantation. Hepatology,51: 1869-1884.
- Fiegel HC,Kaufmann PM,Bruns PM,Kluth D,Horch RE,et al. (2008) Hepatic Tissue Engineering: From transplantation to customized cell-based liver directed therapies from the laboratories. J Cell Mol. Med,12(1): 56-66.
- Michalepaulos GK (2007) Liver Regeneration. J Cell Physiol,213: 286-300.
- Nussler A,Konig S,Ott M,Sokal E,Christ B,et al. (2006) Present status and prespective of cell-based therapies for liver diseases. J of Hepatal,45: 144-159.
- Hegstler JG,Brulpert M,Schormann W,Bauer A,Hermes M,et al. (2005) Generation of human hepatocytes by stem cell technology: definition of the hepatocyte,Expert opin Drug Metab Toxicolgy,1: 61-74
- Synkers S,De Kock J,Rogrers V,Vanhafcke T (2009) In vitro Differentiation of embryonic and adult stem cells onto hepatocytes: State of the Art. Stem cells,27: 577-605.
- Stock P,Steage MS,Muller LP,Sqodda M,Volker A,et al. (2008) Hepatocytes derived from adult stem cells.Transplantation Proceedings,40: 620-623.
- Parekkadan B,Van Pon D,Suganuma K,Cater EA,Bethiaume F,et al. (2007) Mesenchymal stem cell-derived molecules reversr fulminant Hepatic failure. PLoS ONE,2:e941.
- Banas A,Teratani T,Yamamoto Y,Tokuhara M,Takeshita F,et al. (2007) Adipose Tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology,46: 219-228.
- Cholc H,Parashusama N,Park EYH,Suganuma K,Nahanias Y,et al,(2008) Homogenous differentiation of hepatocyte-like cells from embryonic stem cells: Applications for the treatment of acute failure. FASEBJ,22: 898-909.
- Khurana S,Mukhopadhyay A (2008) In vitro Transdifferentiation of adult Hematopiotic stem cells: An alternative source of engraftable hepatocytes. J Hepatology,49: 998-1007.
- Blanc KL,Ringden O (2007) Immunomodulation of mesenchymal stem cells and clinical experience. J Intern Med,262: 509-525.
- Kuot K,Hung SP,Chuang CH,Chen CT,Shih YR,et al. (2008) Stem cell therapy for liver disease: Parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology,134: 2111-2121.
- Jones EA,Kinsey SE,English A,Jones RA,Straszynski L,et al. (2002) Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis and Rheumatism,46: 3349-3360.
- Bianco P,Riminucci M,Granthco S,Robey PG (2001) Bone marrow stromal stem cell. Nature,biology and potential applications. Stem Cells,19: 180-192.
- Shi S,Gronthos S (2003) perivascular niche of postnatal mesenchymal stem cells in human bone marrow and pulp. J Bone Miner Res,18: 696-704.
- Castro-Malaspina,Gay RE,Resnick G,Kapoor N,Meyers P,et al. (1980) Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood,56: 289-301.
- Shi IM (1999) The role of CD146 (Mel-CAM) in biology and pathology. J pathol,189: 4-11.
- Maniatoopoulos C,Sodek J,Melcher AH (1998) Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res,254: 317-330.
- Talens-Visconti R,Bonra A,Jover R,Mirabet V,Carbonell F,et al. (2006) Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol,12: 5834-5845
- Theise ND,Nimmakayalu M,Gardner R,Illei PB,Morgan G,et al. (2000) Liver from bone marrow in humans. Hepatology,32: 11-16.
- Synkers S,Vnhaeaeccke T,Papeleu P,Luttun A,Jiang Y,et al,(2006) Sequential exposure to cytokines reflecting embryogenesis: the key for in vitro differentiation of adult bone marrow stem cells into functional hepatocyte-like cells. Toxicological Science,94: 330-341.
- Aurich I,Mueller LP,Aurich H,Luetzkendorf J,Tisljar K,et al,(2007) Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. GUT,56: 405-415.
- Jozefezuk J,Stachelscheid H,Chavez L,Bioinf D,Hering R,et al. (2010) Molecular characterization of cultured adult liver progenitor cell. Tissue Eng Part C,16: 821-834.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences