Effect of Nitrogen-containing Compounds on Growth Characteristic of the Oleaginous Microalga Chlorella ellipsoidea SD-0701
Lili Liu, Wenyu Luo, Yichen Zhang, Jing Zhuang1, Fujie Zhang
1College of Life Science,Tianjin Normal University,393 binshuixidao Road,Xiqing District,Tianjin 300387,China
2Tianjin Key Laboratory of Animal and Plant Resistance,Tianjin,300387,China
3State Key Laboratory of Plant Genomics,Chinese Academy of Sciences,Beijing 100101,China
Abstract
Microalgae have been the focus of intensive global research over the past few years because their great advantages as a new biomass source for biofuel production. In this study, we investigated the cellular content of major biomolecules in Chlorella ellipsoidea SD-0701 including total lipids, protein and chlorophyll in response to different nitrogen-containing compounds and characterized effects on the cell metabolism. The results indicated that complex nitrogen-containing compounds in the media were more nutritional than simple nitrogen-containing compounds, and yeast extract is the optimum nitrogen-containing compound for the growth of C. ellipsoidea SD-0701. When the concentration of yeast extract was increased from 0 g L-1 to 6 g L-1, chlorophyll and the expression of intracellular soluble proteins were also increased linearly, especially the expression of the 48 kDa ammonium transporter and the 47.4 kDa GTPase. And the biomass and lipid yield were also increased with the concentration of yeast extract added in medium. This study suggest that nitrogen sources were provided to improve the content of cellular solute protein and chlorophyll in C. ellipsoidea SD 0701 so that high algae biomass can be achieved. The concentration of nitrogen source was one of the most important factors for the high algae biomass.
Keywords
Chlorella ellipsoidea; Growth characteristic; Lipid accumulation; Nitrogen-containing compounds.
1. Introduction
As the development of renewable and eco-friendly sources of energy as alternative energy is urgently needed [1],microalgae have gained a lot of attention as a new biomass source for biofuels production over the past few years [2-4]. Algae grow incredibly fast,doubling their numbers every few hours,can be harvested daily,and have the potential to produce a volume of biomass many times greater than the most productive land crops [5]. The lipid content of algae is very high,and under certain conditions some algal species can accumulate up to 70% of their dry weight as oil [6]. In addition,the efficiency of these photosynthetic algae in converting carbon dioxide into carbon-rich lipids,which is only a step or two away from biodiesel. The high oil content together with rapid biomass production makes algae an excellent source for biodiesel production [7].
Several high oil producing microalgae strains such as Isochrysis zhangjiangensis [8],Haematococcus pluvialis [9] and Scenedesmus rubescens [10] have been suggested as potential candidates for biodiesel production. However,production of biofuel from microalgae is dependent on the microalgal biomass production rate and lipid content. Both biomass production and lipid accumulation are limited by several factors,of which nutrients play a key role. To improve the algae biomass and lipid content,some papers have researched on the optimization of carbon sources [11,12] and nitrogen sources [13-15] in media for algae. Mineral elements,such as phosphorus [16] and iron [17],were also reported to be the impact factors.
Nitrogen sources such as ammonium and nitrate couple with either limitation or starvation culture regimes have been identified as the most critical culturing condition affecting lipid metabolism in many microalgae [18,19]. The sensitivity characteristics of microalgae towards changes in culture conditions are expected to cause major drawback in production of suitable oil with consistent fatty acid profile for conversion to high quality biodiesel [20]. In this study,C. ellipsoidea was cultivated in the media containing different nitrogen-containing compounds. The aim was to investigate the total oil,protein and chlorophyll content in response to different nitrogen treatments at early growth phase. We also analyzed the effects of these factors on the cell metabolism of C.ellipsoidea,and provided scientific basis for production microalgae biofuels.
2. Materials and Methods
Oleaginous microalga
Chlorella ellipsoidea SD-0701: Chlorella ellipsoidea SD-0701 was screened from YAOSHI Lake and maintained in the microbiology laboratory of Tianjin Normal University.
Growth medium
SE medium: 0.25 g L-1 NaNO3,0.075 g L-1 K2HPO4·3H2O,0.075 g L-1 MgSO4·7H2O ,0.025 g L- 1 CaCl2·2H2O,0.175 g L-1 KH2PO4,0.025 g L-1 NaCl,0.005 g L-1 FeCl3·6H2O,40 ml soil solution,1 ml Fe- EDTA,1 ml A5 solution,958 ml ddH2O [21]. The medium was sterilized by autoclave at 121°C for 20 min.
Determination of microalgal biomass
1.23×109 microalgal cells were inoculated in each 250-ml flask containing 100 ml medium. All the experiments were kept under continuous illumination at a light intensity of 60–80 μmol m-2s-1 at 25°C for 7 days,and were shaken once every 4 hours (Illuminated incubator,LRH-250Z,Guangdong Province Medical Devices Factory). The algal cells were then harvested from the culture and centrifuged at 5,000 r min-1 for 10 min at 4°C to produce a cellular pellet. The cellular pellet was dried in incubator at 80°C until constant weight was reached,and the microalgal biomass was quantified.
Determination of the lipid content in the microalgal cells
To make a standard curve,total lipids were extracted from 10g microalgae powder by acid hydrolysis method [22]. Lipids of 0.0,0.1,0.2,0.5,1.0,2.0 and 5.0 mg were weighed and they were dissolved in 1 ml chloroform,respectively. 1 ml of H2SO4 was added into each chloroform solution,mixed thoroughly and incubated for 10 min at 100°C. The solutions were cooled,and 5 ml of vanillinphosphoric acid reagent (0.1978 g vanillin,20 ml distilled water,80 ml 85% phosphoric acid) was added into the solutions,respectively. The tubes were then incubated for 2h at room temperature. The OD528 of each chloroform solution was read at 528 nm using a spectrophotometer [23]. The standard curve was plotted with the weight of the lipid on the x axis and the OD528 readings on the y axis,and linear regression analysis was performed on the standard curve.
The algal cells were harvested from 100 ml culture and centrifuged at 5,000 r min-1 for 10 min at 4°C. Cellular deposit was frozen at −80°C (Ultra-low Freezer,UF 3410,Heto-Holten) and thawed for 5 times in 24h,and dried at 80°C until constant weight was reached to produce dry microalgae powder. To determine the lipid content,1 mg of dry microalgae powder was weighed and was treated according to the vanillin colorimetric method. Then lipid yields were calculated. The lipid content is the lipid weight per mg of dry microalgae powder. The lipid yield is the lipid weight per liter of the cultured-alga media.
The comparison method for microalgae growth and lipid accumulation in media supplemented with different nitrogen-containing compounds
The Basic Medium (BM) was SE medium without NaNO3. The effects of microalgae growth were tested by adding different nitrogen-containing compounds into the BM. The compounds were KNO3 2.94 mmol L-1,Ca(NO3)2 2.94 mmol L-1,(NH4)2SO4 2.94 mmol L-1,tryptone 3 g L-1,soybean flour 3 g L-1 and yeast extract 3 g L-1. The control group was SE medium without any nitrogencontaining compounds. The microalgal biomasses and lipid contents were measured according to the above methods,the lipid yields of cultures were calculated according to the lipid contents. Each experiment and the subsequent analysis were repeated three times.
Determination of intracellular soluble protein in microalgal cells
Protein standard was prepared with Bovine serum albumin (BSA) with a concentration series of 20,40,60,80 and100 μg mL-1. 5 ml of Coomassie Brilliant Blue reagent (60 mg Coomassie Brilliant Blue G- 250,100 ml of 3% perchloric acid) was added into each tube containing 1 ml of BSA standard solution and mixed thoroughly. One group was treated with the distilled water as a control. The tubes were then incubated for 15 min at room temperature and the absorbance was read at 595 nm using a pectrophotometer. Plot the standard curve with the protein concentrations on x axis and the OD528 readings on y axis.
Then the intracellular soluble protein content of microalgae were determined as follows: after culturing for 4 days,microalgal cells were disrupted and the supernatant was obtained by centrifugation at 10000 r min-1 for 20 min. Mix 1 ml supernatant with 5 ml Coomassie Brilliant Blue reagent. The mixtures were incubated for 15 min at room temperature and the absorbance was measured at 595 nm using a pectrophotometer. The intracellular soluble protein content of microalgae was calculated according to the standard curve. Each experiment and the subsequent analysis were repeated three times.
SDS-PAGE
The cells were collected from the cultured-alga media by centrifugation (6,000r min-1,10 min,4°C),resuspended in 5 ml of PBS,and sonicated on ice with a tip sonicator 60 times for 3s each. After sonication,the sample buffer was added,and the sample was boiled for 10 min and centrifuged in a microcentrifuge. The supernatant was transferred to a new microcentrifuge tube. Gel electrophoresis was carried out as described in Olkkonen and Bamford [24]. The gels were scanned with Gel imaging system (Gel imaging system,GelDoc XR+,Bio-Rad Laborateries). Protein patterns were analyzed by using an image-processing apparatus (Quantity one V4.62).
Method for identifying proteins
After SDS-PAGE,the protein patterns were cut off from gel for identification. The protein was identified by peptide mass fingerprinting (PMF). All peak sets were scored,using the Mascot tool from Matrix Science.
Determination of chlorophyll content
80% acetone are used to extract the pigments from the separated algal cells. Centrifuge 1.05×108 algal cells at 3000 r/min for 1 min and discard the supernatant. Wash the pellet three times with PBS and then resuspended into 5 ml of 80% acetone. Extract the pigment at 4°C for 24h and then centrifuged. The chlorophyll content is calculated by reading the absorption at 663nm and 645nm of the pigment extract in a spectrophotometer and use the following equation:
Chlorophyll content=8.02×OD663+20.2×OD645.
Statistical analysis
All the experiments were conducted in three replicates. The data were analyzed using one-way analysis of variance (ANOVA) with SPSS software (version 18.0). A confidence of 95 % (probability limit of p < 0.0 5) was chosen.
3. Results
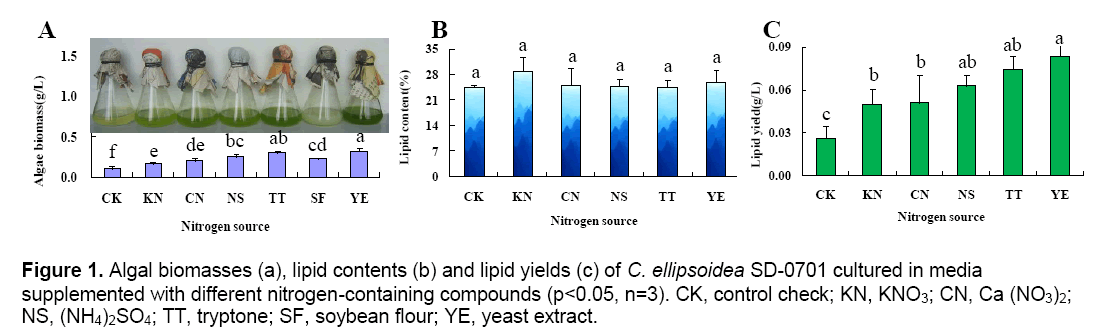
Different nitrogen-containing compounds in the media have different availability,so there are significant differences in the growth rate and lipid accumulation of microalgae,which leads to different microalgal biomasses and lipid contents. The results were shown in Figure 1.
Figure 1A showed that the cell concentration in flasks with different cultured-alga media was not same. The highest cell concentration was the group cultured in media with yeast extract,and the color was deep green. The lowest was the group cultured in media without any nitrogen-containing compounds,and the color was light green. The soybean flour was obtained after soybean was directly grinded,and then added into media,so the cultured-alga media of this group was white finally. The determination results indicated microalgal biomasses of C. ellipsoidea SD-0701 cultured in media with nitrogen-containing compounds were much higher than that of the control group. In this experiment,complex nitrogen-containing compounds in the media were greater impact than simple nitrogen-containing compounds to promote C. ellipsoidea SD-0701 growth. Among the tested complex nitrogen-containing compounds,yeast extract (0.32 g L-1) was the optimum nitrogencontaining compound,followed by tryptone (0.30 g L-1) and soybean flour (0.23 g L-1).
The lipid content of each treatment was quantified according to the standard curve of the lipid content y = 4.694 x,R2= 0.993 (x: the weight of lipid (mg),y: OD528). The results indicated that the lipid content of each treatment had no significant differences (Fig. 1B). The lipid yields (Fig. 1C) of cultured-alga media were calculated according to the lipid contents of the microalgae powder. The lipid yield of C. ellipsoidea SD-0701 was markedly affected by the nitrogen-containing compounds in the media (Fig. 1C). The highest lipid yield (83.2 mg L-1) was achieved in the medium containing yeast extract. The lipid yield of the sample supplemented with soybean flour was not determined because soybean flour itself contains lipids.
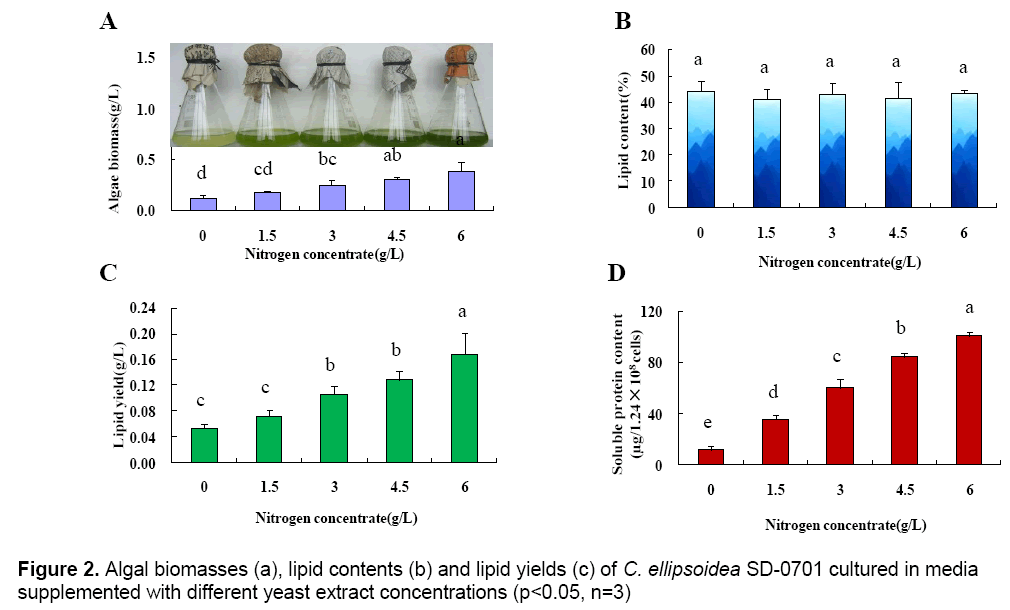
The above results indicated that yeast extract was the optimum nitrogen-containing compound for C. ellipsoidea SD-0701. The effect of the yeast extract concentration on the growth of C. ellipsoidea SD-0701 was then investigated. The concentrations of yeast extract were set at 0,1.5,3,4.5 and 6 g L-1 and microalgae were cultivated in media with these concentrations of yeast extract. The microalgal biomasses,lipid contents and lipid yields were determined (Figure 2 A-C).
With yeast extract concentration increased from 0 g L-1 to 6 g L-1,the microalgal biomass increased linearly (Fig. 2A),showing a positive correlation between nitrogen concentration and microalgal biomass. The lipid contents of microalgae powder were 44.43 % (0 g L-1),41.27 % (1.5 g L-1),43.23 % (3 g L-1),41.74 % (4.5 g L-1) and 43.55 %(6 g L-1). There were no significant differences between each treatment. As shown in Fig. 2C,the lipid yield per liter cultured-alga media increased gradually with the increase of microalgal biomass.
To better understand the effect of yeast extract on microalgal growth,the intracellular soluble protein content of 1.28×108 microalgal cells was determined after a 2-day incubation. The intracellular soluble protein content were 12.14 μg (0 g L-1),35.42 μg (1.5 g L-1),59.96 μg (3 g L-1),84.54 μg (4.5 g L-1) and 100.48μg (6 g L-1),respectively (Fig. 2D). The result indicated that the content of intracellular soluble protein increased gradually with increasing concentrations of yeast extract,which was consistent with the change in microalgal biomass.
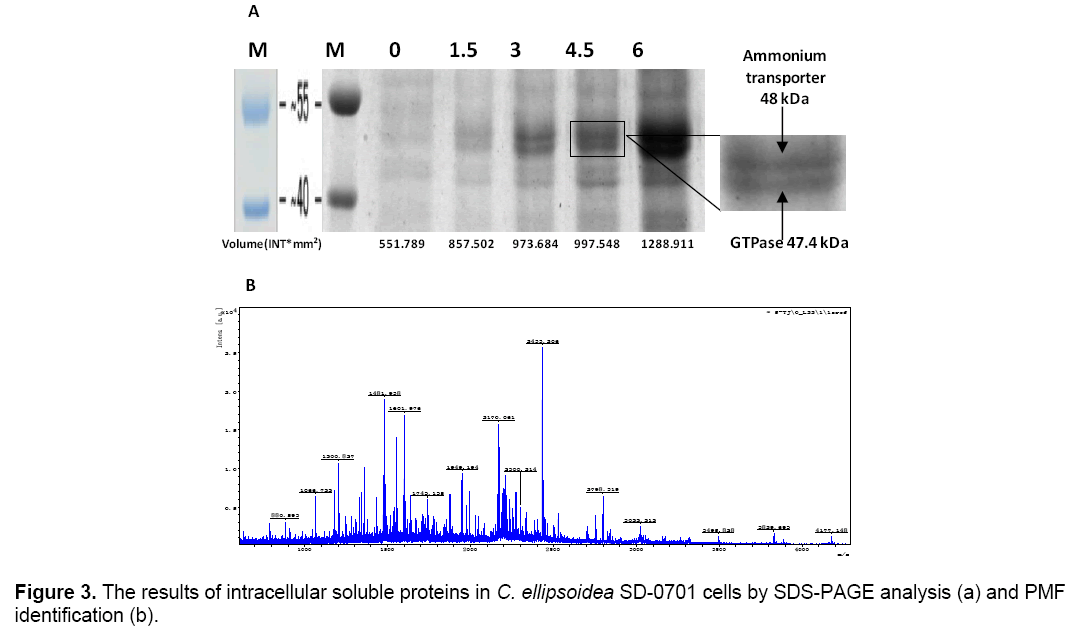
The intracellular soluble proteins in C. ellipsoidea SD-0701 cells were analyzed to better understand the mechanism of the effect of yeast extract. Same numbers of microalgal cells were sonicated after a 2-day incubation and the whole-cell protein were characterized by SDS-PAGE. Coomassie brilliant blue staining of the gel is shown in Figure 3 A. Lane M was molecular weight of protein Marker. Lane 1,2,3,4 and 5 proteins isolated from five treatments with yeast extract concentrations of 0,1.5,3,4.5 and 6 g L-1. Two bands of 48 and 47.4 kDa were visible in each lane and their intensity increased gradually with increased concentrations of yeast extract. Protein patterns were analyzed by Quantityone software V4.62. The volumes of the bands were about 551.78 INT*mm2 (0 g L-1),857.50 INT*mm2 (1.5 g L-1),973.68 INT*mm2 (3 g L-1),997.54 INT*mm2 (4.5 g L-1),1288.91 INT*mm2 (6 g L-1),respectively. This result indicated that the yeast extract in the medium could induce the expression of two proteins of 48 and 47.4 kDa,and the effect of the induction was positively correlated with the concentration of yeast extract.
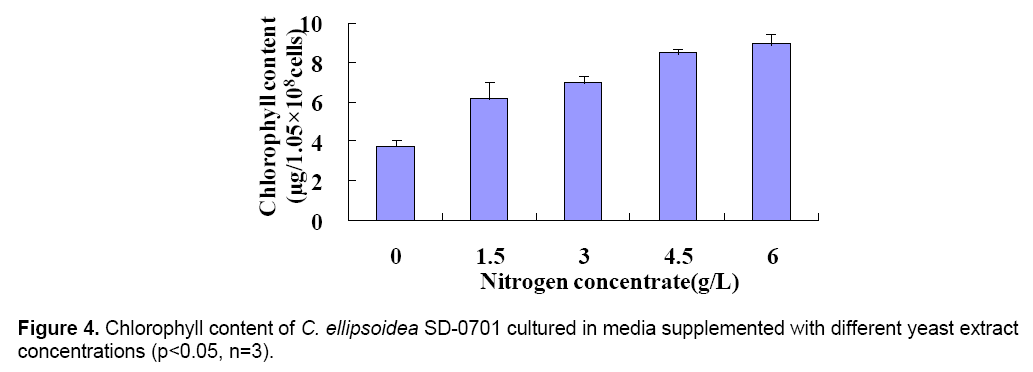
The 47.4 kDa protein was identified by peptide mass fingerprinting (PMF). All peak sets were scored using the Mascot tool from Matrix Science. The identified protein was GTPase (Figure 3 B). The chlorophyll content was determined after culture for 6 days. And the chlorophyll content of treatments were 3.76(0),6.18(1.5),7.03(3),8.49(4.5) and 8.95(6) μg/1.05 × 108 cells,respectively (Figure 4 ). With the yeast extract concentration increasing,the absorbed nitrogen increased. Except for growth,most of the remaining absorbed nitrogen was used for chlorophyll systhesis.
4. Discussion
Nitrogen-containing compounds provide nitrogen source for cell growth. In addition,complex organic nitrogen-containing compounds also provided extra amino acids,vitamins and growth factors. The microalgal biomasses cultured in media supplemented with complex nitrogen-containing compounds were 0.32 g L-1 (yeast extract),0.30 g L-1 (tryptone) and 0.23 g L-1 (soybean flour). Another reason for this result is that the amino nitrogen accounts for 5.1% of yeast extract and 2.5% of typtone,and the content of amino nitrogen in soybean flour is lower. According to previous experiences,ammonium nitrogen is easier to enter the C. ellipsoidea SD-0701 cells than nitrate nitrogen is,and moreover,it can be synthesized into glutamine by glutamate synthase in the cells,which can lead to rapid protein synthesis [25]. So yeast extract was the optimum nitrogen-containing compound among the tested nitrogen-containing compounds.
C. ellipsoidea SD-0701 were cultured with different concentrations of yeast extract. Determined the content of intracellular soluble protein of each treatment and it indicated that the yeast extract in the medium can contribute to the increase of the intracellular soluble protein content in a dose dependent manner (Figure 2 D). The content of intracellular soluble protein correlates with the amount of enzymes that participate in the biocatalytic reactions. The higher the content of intracellular soluble protein is,the more enzymes there are participating in metabolism. This promotes cells to synthesize organic substance to support rapid growth.
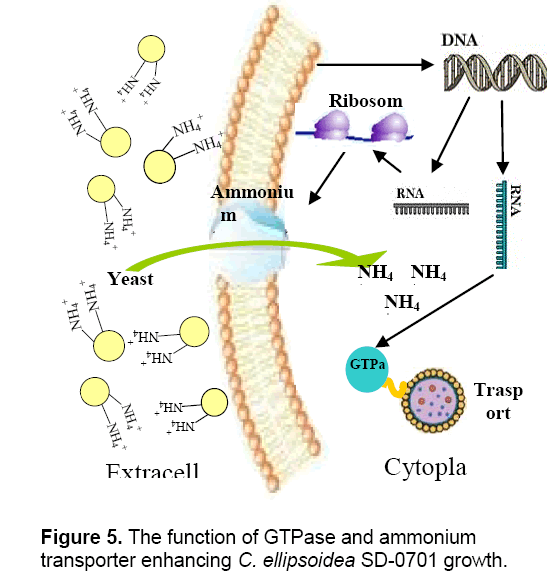
Valenzuela et al. [25] reported that the upregulation of nitrogen metabolism genes including ammonium and nitrate transporters is most likely a result of the fast growth and biomass accumulation during exponential growth. The whole-cells’ proteins were characterized by SDS-PAGE. According to the analysis of Quantityone software V4.62 and peptide mass fingerprinting (PMF),the expression of ammonium transporter with mol. masse of 48kDa [26] and GTPase with mol. mass of 47.4 kDa increased with the increased concentrations of yeast extract. Ammonium transporter is an active transport carrier of ammonium ion which is widely present in cell membrane of microorganisms,plant and animal cells (Figure 5 ). Nguyen et al. [27] reported that GTPases were identified in Chlamydomonas reinhardtii by proteomics,and they were potentially involved in vesicle trafficking and transport. GTPases function as molecular switches that cycle between an active GTP-bound state and an inactive GDP-bound state. They regulate vesicular transport by controlling actin filament assembly and organization [28].
GTPase accelerates the substance transport among membrane-enclosed organizations in cell,which promote the growth and metabolism of C. ellipsoidea SD-0701. The higher content of yeast extract could induce the overexpression of ammonium transporter and GTPase which led to the yeast extract entering cells increased,so C. ellipsoidea SD-0701 grew rapidly (Figure 3 A).
In conclusion,complex nitrogen-containing compounds in the media were more nutritional than simple nitrogen-containing compounds,and yeast extract is the optimum nitrogen-containing compound for the growth of C. ellipsoidea SD-0701. When the SE medium was supplemented with suitable concentration of yeast extract,the microalgal biomass and lipid yield increased 3.05 and 3.21 times,respectively. When the concentration of yeast extract was increased,chlorophyll and the expression of intracellular soluble proteins were also increased linearly,especially the expression of the 48 kDa ammonium transporter and the 47.4 kDa GTPase. And the biomass and lipid yield were also increased with the concentration of yeast extract. This study suggest that nitrogen sources were provided to improve the content of cellular solute protein and chlorophyll in C. ellipsoidea so that high algae biomass and lipid yield can be achieved. The concentration of nitrogen source was one of the most important factors for the high algae biomass.
Acknowledgments
This work was supported in part by the Natural Science Foundation of China under grant (#31172029),Tianjin Natural Science Foundation under grant (#07JCYBJC15400),and a grant (#0802220) from Tianjin Agriculture and Rural Affairs Committee,as well as a grant (#12ZCZDNC00600) from Tianjin Municipal Science and Technology Program.
References
- Tushar KG,Mark AP (2011) Bioenergy,In: Energy Resources and Systems,pp 327-418.
- Satyanarayana KG,Mariano AB,Vargas JVC (2011) A review on microalgae,a versatile source for sustainable energy and materials. Int J Energy Res 35: 291–311.
- Nwachukwu AN,Chukwu MA (2012) The potential of macroalgae as a source of carbohydrates for use in bioethanol fermentation. Int J Energy Envir 3: 761- 774.
- Wu YH,Hu HY,Yu Y,Zhang TY,Zhu SF,Zhuang LL,Zhang X,Lu Y (2014) Microalgal species for sustainable biomass/lipid production using wastewater as resource: A review. Renew Sust Energy Rev 33: 675-688.
- Dismukes GC,Carrieri D,Bennette N,Ananyev GM,Posewitz MC (2008) Aquatic phototrophs: efficient alternatives to land-basedcrops for biofuels. Curr Opin Biotech 19: 235-240.
- Perego C,Ricci M (2012) Diesel fuel from biomass. Catal Sci Technol 2:1776-1786.
- Demirbas (2010) Biodiesel from oilgae,biofixation of carbon dioxide by microalgae: A solution to pollution problems. Appl Energy 88: 3541-3547.
- Feng D,Chen Z,Xue S,Zhang W (2011) Increased lipid production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement. Bioresour Technol 102: 6710-6716.
- Damiani MC,Popovich CA,Constenla D,Leonardi PI (2010) Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour Technol 101: 3801-3807.
- Lin Q,Lin J (2011) Effect of nitrogen source and concentration on biomass and oilproduction of a Scenedesmus rubescens like microalga. Bioresour Technol 102:1615-1621.
- Li Z,Yuan H (2011) Optimization of the biomass production of oil algae Chlorella minutissima UTEX2341. Bioresour Technol 102: 9128-9134;
- Lin Q,Gu N (2012) Effects of inorganic carbon concentration on carbon formation,nitrate utilization,biomass and oil accumulation of Nannochloropsis oculata CS 179. Bioresour Technol 111: 353-359.
- He PJ,Mao B (2013) Cultivation of Chlorella vulgaris on wastewater containing high levels of ammonia for biodiesel production. Bioresour Technol 129:177-181.
- Ruangsomboon S,Ganmanee M,Choochote S (2013) Effects of different nitrogen,phosphorus,and iron concentrations and salinity on lipid production in newly isolated strain of the tropical green microalga,Scenedesmus dimorphus KMITL. J Appl Phycol 25: 867-874.
- Kothari R,Pathak VV (2012) Experimental study for growth potential of unicellular alga Chlorella pyrenoidosa on dairy waste water: an integrated approach for treatment and biofuel production. Bioresour Technol 116: 466-470.
- Chen M,Tang H (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102:1649-1655.
- Yeesang C,Cheirsilp B (2011) Effect of nitrogen,salt,and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour Technol 102: 3034-3040.
- Xu N,Zhang X,Fan X,Han L,Tseng CK (2001) Effects of nitrogen source and concentration on growth rate and fatty acid composition of Ellipsoidion sp.(Eustigmatophyta). J Appl Phycol 13: 463-469.
- Hu Q,Sommerfeld M,Jarvis E,Ghirardi M,Posewitz M,Seibert M,Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54: 621-639.
- Cha TS,Chen JW,Goh EG,Aziz A,Loh SH (2011) Differential regulation of fatty acid biosynthesis in two Chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application. Bioresour Technol 102:10633-10640.
- Zhang CM,Pan WB,Chen YZ (2009) The Toxic Effects of the Extracellular Active Components from One Algae-lysing Bacteria on Chlorella Pyrenoidosa. Microbiology 36: 821-825.
- Ma S,Fu LL,Wang M,Hu XW,Tan DG,Zhang JM (2010) Comparision of extraction methods for crude fat from microalgae. China Oils And Fats 35: 77-79.
- Gunnel A,Leonardo M (1991) Lipid analysis of freshwater microalgae: A method study. Arch Hydrobiol 121: 295-306.
- Olkkonen V,Bamford D (1989) Quantitation of the absorption and penetration stages of bacteriophage 6 infection. Virology 17: 229-238.
- Valenzuela J,Mazurie A,Carlson RP,Gerlach R,Cooksey KE,Peyton BM,Fields MW (2012) Potential role of multiple carbon fixation pathways during lipid accumulation in Phaeodactylum tricornutum. Biotechnol Biofuels 5:40.
- Luo YY,Liu SK (2009) Research Progress of Ammonium Transporter in Plants. Genomics Appl Biol 28: 373-379.
- Nguyen HM,Baudet M,Cuine S et al. (2011) Proteomic profiling of oil bodies isolated from the unicellular green microalga Chlamydomonas reinhardtii: With focus on proteins involved in lipid metabolism. Proteomics 11: 4266-4273.
- Bishop AL,Hall A (2000) Rho GTPases and their effector proteins. Biochem 348: 241-255.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences