Dissociation of Protein Aggregates by Mid-infrared Laser
Takayasu Kawasaki, Kazuhiro Nakamura
Takayasu Kawasaki1 and Kazuhiro Nakamura2,*
1IR Free Electron Laser Research Center, Research Institute for Science and Technology, Organization for Research Advancement, Tokyo University of Science, 2641, Yamazaki, Noda, Chiba 278-8510, Japan;
2Department of Laboratory Sciences, Gunma University Graduate School of Health Sciences, 3-39-22, showa-machi, Maebashi, Gunma 371-8511, Japan.
Received date: June 04, 2016; Accepted date: July 21, 2016; Published date: July 28, 2016
Citation: Kawasaki T, Nakamura K. Dissociation of protein aggregates by mid-infrared laser. Electronic J Biol, 12:4
Abstract
Amyloid fibrils are insoluble fibrillar deposits consisting of beta sheet proteins that accumulate in various tissues. The pathogenesis of some forms of amyloidosis is closely related to amyloid fibrils. For example, amyloid beta protein fibrils are mainly found as extracellular deposits in the brains of individuals with Alzheimer’s disease, which lead to neuronal cell death. Therefore, reducing the fibrils is a major target for treating amyloidosis. Currently, there are no effective drugs for amyloidosis, yet treatments have been explored using other biomedical approaches. In addition to biomedical approaches, engineering techniques have also been applied to this problem. Among them, recent papers have suggested a Free- Electron Laser (FEL) with a wavelength set in the midinfrared region could dissociate protein aggregates. The FEL is effective for dissociating aggregates rich in α-helix and β-sheet structures. This approach might lead to novel therapies for amyloidosis.
Keywords
Amide band; Amyloid fibrils; Freeelectron laser; Protein aggregates.
1. Introduction
The protein aggregation process often induces a reduction of normal protein functions, such as enzymatic activities, accompanied by a decrease in water-solubility. As a result, protein aggregates are closely associated with several serious diseases. In amyloidosis, amyloid fibrils are formed by multiple proteins and are deposited in the tissues of various organs [1]. In the brain, aggregation of amyloid β, tau and polyglutamine-containing proteins are the primary cause of Alzheimer’s disease and polyglutamine diseases [2,3]. Also, α-synuclein and prions can form aggregates in the brain [4,5]. In tissues outside of the brain, lysozyme, insulin, calcitonin, lactoferrin, β2-microglobulin, and immunoglobulin light and heavy chains can also form aggregates [6-11]. These fibrils are commonly rich in β-sheet structures and are insoluble in physiological solutions, whereas the primary structure of each fibril is different. On the other hand, keratin protein, which is a major structural component of the cytoskeleton in dermal tissues, is known to be a different kind of aggregate-forming protein [12]. Keratin tends to form non-covalent assemblies during aging, and the aggregation process causes hair damage and several skin diseases. Keratin is comparatively large at approximately 50 kDa, and the aggregate is typically rich in α-helix [13].
The detailed mechanism by which aggregates form is not fully understood, and therefore treatments for aggregate-associated diseases have not been established yet. Theoretically, cellular dysfunctions caused by the aggregates should be ameliorated by reducing the amount of protein aggregates found in pathological tissues. However, synthetic protein aggregates are robust structures under physiological conditions and can be disaggregated in the presence of denaturants, such as guanidine hydrochloride and organic solvents (e.g. dimethyl sulfoxide). These reagents are usually toxic for cells and are therefore not suitable for the treatment of diseases.
A polypeptide generally consists of several amide bonds that connect an amino acid to the next amino acids by releasing one molecule of water. The amide bond is composed of C=O, N-H, and C-N bonds, and each chemical bond exhibits a specific vibration mode, including amide I (C=O stretch), amide II (N-H bending), and amide III (C-N stretch). A strong infrared absorption is especially observed at the amide I band [14,15].
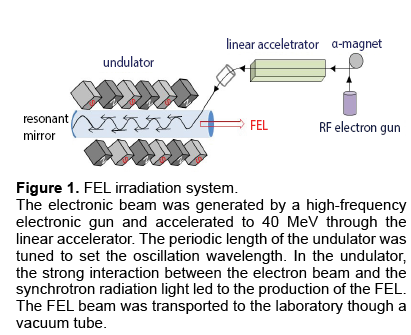
We have recently discovered that several protein aggregates can absorb most photon energy at the amide I band, and the aggregate structure of proteins can be deformed by a Free-Electron Laser (FEL) tuned to the amide bands [16-20]. The FEL in our facility is a high-peak powered and pico-second pulsed laser and has a tunable wavelength within the mid-infrared region (5-10 μm). The oscillation system is shown in Figure 1. An electron beam generated by a high-frequency RF electron gun (2,856 MHz) was injected into an undulator (a periodic magnetic field) through an α-magnet and a linear accelerator to generate Synchrotron Radiation (SR). The SR was amplified between a pair of mirrors positioned at both sides of the undulator through interaction with the electron beam, which produced a coherent laser light. The power density of the FEL was estimated to be 35–45 mJ/cm2, and the time structure of the FEL was composed of macro- and micro-pulses in which one micro-pulse of 2 ps width was separated by a 350 ps interval and one macro-pulse of 2 μs duration was structured by about six thousands of micro-pulses.
Figure 1: FEL irradiation system.
The electronic beam was generated by a high-frequency electronic gun and accelerated to 40 MeV through the linear accelerator. The periodic length of the undulator was tuned to set the oscillation wavelength. In the undulator, the strong interaction between the electron beam and the synchrotron radiation light led to the production of the FEL. The FEL beam was transported to the laboratory though a vacuum tube.
In this study, we present examples in which a nonamyloid aggregate (keratin) and an amyloid fibril (insulin) were efficiently converted to their nonaggregate forms by FEL irradiation. Furthermore, we discuss a possible mechanism underlying the effect.
1.1 Irradiation effect on non-amyloid protein aggregates
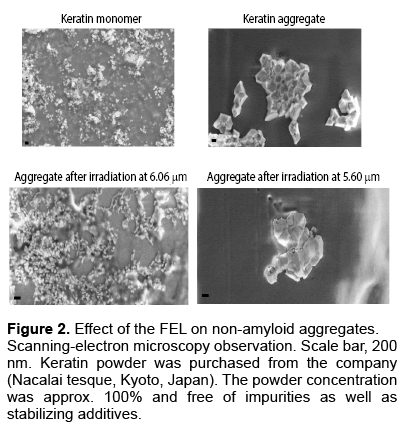
We sought to test the usefulness of the FEL to dissociate non-amyloid aggregates. To this end, we chose a keratin aggregate [20]. Scanning-Electron Microscopy (SEM) observations showed that many particles that were approximately one hundred nanometers in diameter were keratin monomer samples, whereas a larger solid was the aggregate (Figure 2). The irradiation by FEL at wavelength 6.06 μm (amide I) changed the angular solid to a non-ordered assembly consisting of a number of small particles. In contrast, at wavelength 5.60 μm, the aggregate solid remained although its angular surface changed to a smooth surface. These observations indicated that the aggregate structure was dissociated by FEL irradiation at the amide I band. Secondary structure analysis indicated that the α-helix content in the aggregate structure decreased to a level almost comparable to the level in the monomer state after irradiation at 6.06 μm, whereas the keratin aggregate before irradiation was rich in α-helix [20]. Both irradiations at 6.51 μm (amide II band) and 8.06 μm (amide III band) were partially effective but were effective to a lesser extent than irradiation at the amide I band.
1.2 Irradiation effect on amyloid fibrils
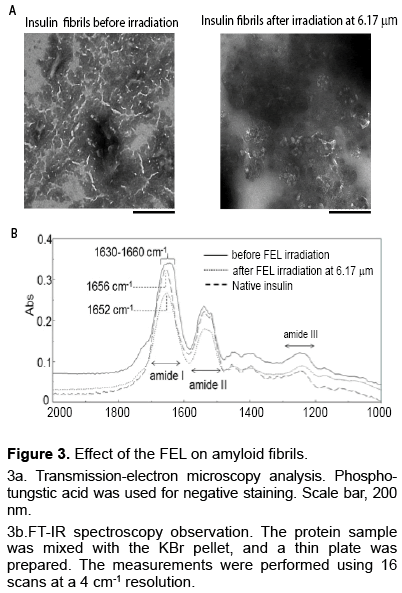
Similar to the dissociation of α-helix structures, β-sheet structures were also dissociated by the same wavelength from the FEL [17]. Amyloid fibrils of insulin peptides were prepared in an acidic solution (20% acetic acid). The resulting fibrils were spotted on the slide base and subjected to irradiation. After irradiation by the FEL, the fibril structure was analyzed using transmission-electron microscopy (Figure 3a) and FT-IR spectroscopy (Figure 3b). The insulin fibrils were observed as tiny strings, and the lengths of the fibrils ranged from one hundred nanometers to two hundred nanometers. These strings decreased substantially after irradiation at 6.17 μm (1620 cm-1), which corresponded to the amide I band. FT-IR spectra of the fibrils showed a broad peak (1,630– 1,660 cm-1) at the amide I band (solid line), although the main peak appeared at 1,656 cm-1 in the native state (dashed line). The β-sheet content in the fibrils was quantified at around 40%, whereas it was about 10% in the native state, as shown in secondary structure analysis of absorbance intensity at the amide I band. After 1 h of irradiation, the broad peak of the fibrils converted approximately to a single peak at 1,652 cm-1 (dotted line) and the β-sheet content was reduced to 25%. These results indicate that a conformational change occurred in the insulin fibrils and that β-sheet rich structures can be reduced by FEL irradiation at the amide I band.
Figure 3: Effect of the FEL on amyloid fibrils.
3a. Transmission-electron microscopy analysis. Phosphotungstic acid was used for negative staining. Scale bar, 200 nm.
3b.FT-IR spectroscopy observation. The protein sample was mixed with the KBr pellet, and a thin plate was prepared. The measurements were performed using 16 scans at a 4 cm-1 resolution.
1.3 Insight into the mechanism of dissociating aggregates with an IR laser
For amyloid fibrils, the aggregation reaction is caused by ionic amino acid residues, such as lysine and glutamic acid, and hydrophobic interactions between aromatic amino acids prompt fibril formation via π−π interactions. Similarly, keratin aggregates are formed by non-covalent bonds, such as hydrogen bonds, and ionic bonds between peptide chains. These non-covalent bonds are easily influenced by heating, and the aggregate structure can be melted by external heating at >80°C [21]. Because we kept the aggregates at 37°C and the increase in temperature of the sample was small during FEL irradiation, the energy accumulation by the FEL likely occurred at the amide bonds of the aggregated protein. This accumulation would be able to drive dissociation of the aggregate structure into a non-aggregate form. In general, thermal diffusion from protein matrices to surrounding water molecules can occur within a 10– 100 ns time scale during the laser ablation of tissue materials. Therefore, successive resonant excitation at amide bonds by pico-second pulses of FEL irradiation may be enough for thermal confinement within the aggregate structure, which led to dissociation of the aggregate. Moreover, we observed that vaporization of water was substantially promoted by FEL irradiation at 6 μm compared to 5 μm. Overall; it can be proposed that FEL irradiation at 6 μm heated both the protein aggregate and surrounding water molecules, which induced dissociation of the aggregate into a nonaggregate form.
1.4 Future perspectives
Although the FEL has mainly been used for physical chemistry and material technology development, it could be also applied to the field of medicine. Lasermediated surgical therapy has been recognized as an effective strategy for tissue ablation and selective removal of injured areas. Using naked proteins, the FEL proved to correct not only non-amyloid aggregated protein but also amyloid fibrils into non-aggregated fibrils. The next step would be to demonstrate the capability of the FEL for reducing aggregates in cells.
Acknowledgement
We thank the staff of the IR-FEL Research Center at Tokyo University of Science for kindly providing beam time. This work was supported in part by the Open Advanced Research Facilities Initiative and Photon Beam Platform Project of the Ministry of Education, Culture, Sport, Science and Technology, Japan.
References
- Sipe JD, Benson MD, Buxbaum JN. (2014).Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid.21: 221-224.
- Stancu IC, Vasconcelos B, Terwel D, Dewachter I. (2014) Models of b-amyloid inducedtau-pathology: The long and “folded” road to understand the mechanism. MolNeurodegener.9: 51.
- Nakamura K, Mieda T, Suto N, Matsuura S, Hirai H. (2015). Mesenchymal stem cells as apotential therapeutic tool for spinocerebellar ataxia.Cerebellum.14:165-170.
- Spillantini MG, Schmidt ML, Lee VMY, et al. (1997).a-Synuclein in Lewy bodies.Nature.388: 839-840.
- Panza G, Stöhr J, Dumpitak C.(2008). Spontaneous and BSE-prion-seeded amyloid formation of full length recombinant bovineprion protein.BiochemBiophys Res Commun.373: 493-497.
- Frare E, Mossuto MF, Polverino de Laureto P. (2006).Identification of the core structure of lysozyme amyloid fibrils by proteolysis.J Mol Biol. 361:551-561.
- Zako T, Sakono M, Hashimoto N. (2009). Bovine insulin filaments inducedby reducing disulfide bonds show a different morphology, secondary structure, and cell toxicity fromintact insulin amyloid fibrils. Biophys J.96: 3331-3340.
- Kamihira M, Naito A, Tuzi S. (2000). Conformational transitions andfibrillation mechanism of human calcitonin as studied by high-resolution solid-state 13C NMR.Protein Sci. 9: 867-877.
- Araki-Sasaki K, Osakabe Y, Fukuoka H. (2014). Findings of secondarycorneal amyloidosis with ultrahigh-resolution optical coherence tomography.ClinicalOphthalmology.8: 2115-2119.
- Lu M, Hiramatsu H, Goto Y, Kitagawa T. (2006). Structure of interacting segments in thegrowing amyloid fibril of ÃÆïÃâÃÂÃââ2-microglobulin probed with IR spectroscopy. J Mol Biol. 362: 355-364.
- Pinney JH, Hawkins PN. (2012). Amyloidosis.Ann ClinBiochem. 49: 229-241.
- Chamcheu JC, Navsaria H, Pihl-Lundin I. (2011). Chemicalchaperones protect epidermolysisbullosa simplex keratinocytes from heat stress-induced keratinaggregation: Involvement of heat shock proteins and MAP kinases. J Invest Dermatol. 131:1684-1691.
- Marchuk D, McCrohon S, Fuchs E. (1985). Complete sequence of a gene encoding a humantype I keratin: sequences homologous to enhancer elements in the regulatory region of the gene.ProcNatlAcadSci USA.82: 1609-1613.
- Bandekar J. (1992). Amide modes and protein conformation.BiochimBiophysActa – ProteinStructure and Molecular Enzymology.1120: 123–143.
- Mantsch H, Jackson M. (1995). Molecular spectroscopy in biodiagnostics (from Hippocrates toHerschel and beyond).JMolStruct.347: 187-206.
- Kawasaki T, Fujioka J, Imai T, Tsukiyama K. (2012). Effect of mid-infrared free-electron laserirradiation on refolding of amyloid-like fibrils of lysozyme into native form.Protein J. 31: 710-716.
- Kawasaki T, Fujioka J, Imai T, Torigoe K, Tsukiyama K. (2014). Mid-infrared free-electronlaser tuned to the amide I band for converting insoluble amyloid-like protein fibrils into the solublemonomeric form. Lasers Med Sci. 29: 1701-1707.
- Kawasaki T, Imai T, Tsukiyama K. (2014). Use of a mid-infrared free-electron laser (MIR-FEL)for dissociation of the amyloid fibril aggregates of a peptide.J Anal Sci Meth Instrum.4: 9-18.
- Kawasaki T, Yaji T, Imai T, Ohta T, Tsukiyama K. (2014). Synchrotron-infrared microscopyanalysis of amyloid fibrils irradiated by mid-infrared free-electron laser.Am J Anal Chem. 5:384-394.
- Kawasaki T, Yaji T, Ohta T, Tsukiyama K. (2016). Application of mid-infrared free-electronlaser tuned to amide bands for dissociation of aggregate structure of protein. J Synchrotron Rad. 23:152-157.
- Morel B, Varela L, Conejero-Lara F. (2010). The Thermodynamic stability of amyloid fibrilsstudied by differential scanning calorimetry.J Phys Chem. B. 114: 4010-4019.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences