Chemosensitivity of Hybridoma Cell Lines to Actinomycin D and Bleomycin Sulfate Compared to Non-hybridoma Cell Lines
N. Jouyan, H. Mozdarani, M. A. Shokrgozar, V. Farahat, B. Saffari
1Human Genetic Research Group, Iranian Academic Center for Education, Culture & Research (ACECR), Fars Province Branch, Shiraz, Iran;
2Institute of Biochemistry and Biophysics (IBB), University of Tehran, Tehran, Iran;
3Department of Medical Genetics, School of Medical Sciences, Tarbiat Modares University, Tehran, Iran;
4National Cell Bank of Iran, Pasteur Institute, Tehran, Iran;
5School of Biology, College of Sciences, University of Tehran, Tehran 14155-6455, Iran.
- Corresponding Author:
- Tel: + 98-21-82883830
Fax: + 98-21-88006544
Email: mozdarah@modares.ac.ir
Abstract
Cytogenetic effects of two clastogenic antitumor agents, actinomycin D and bleomycin sulfate were assessed in G2 phase of cell cycle in two heterohybridoma cells, and two non-hybrid cell lines. Since heterohybridoma cells carry chromosome constitutions from different species, their responses to various environmental stimuli may vary and depend on their chromosomal origins. Two hybridoma cell lines, HF2x653 and F3B6, and two nonhybridoma cell lines, WIL2 and NS-1, as their parents were treated with bleomycin sulfate and actinomycin D in G2 phase of the cell cycle. Metaphase spreads were prepared and various types of chromatid aberrations were scored. The frequencies of chromatid aberrations of hybrid lines in both treatments were between the frequencies of WIL2 and NS-1. HF2 showed same chromosome aberration frequency with WIL2 and F3B6 with NS-1. The results indicated that chemosensitivity of hybridoma cell lines investigated in this study was nearly similar to their parental cell lines.
Keywords
Actinomycin D, Bleomycin Sulfate, Hybrid cell, Chromosome aberration
1. Introduction
Exposure to genotoxic compounds can induce mutations. Bleomycin-sulfate (BLM-S) and actinomycin D (Act-D) are two popular examples of mutagens that also have anticancer activity. BLM-S, an antitumor and antibiotic drug obtained from Streptomyces verticillus, is a radiomimetic agent and, in the presence of iron [Fe (II)] and O2, catalyzes single and double stranded cleavage of DNA [1]. BLM-S does not interfere directly with the DNA replication but acts as an S-independent fashion [2] and depending on the phase of the cell cycle, it produces chromatid-type (in G2 phase) or chromosome-type (in G0 and G1 phase) aberrations.
The actinomycins are a class of polypeptide antibiotics produced by the genus Streptomyces. The most significant member of actinomycins is actinomycin D which is primarily used as an investigative tool in cell biology to inhibit transcription. Act-D does this by making a complex with DNA at the transcription initiation site and thus prevents elongation by RNA polymerase [3]. This occurs largely through its guanine binding capacity [4] which has been found to induce chromosome breakage by Ostertag and Kersten in 1965 [5]
As mentioned previously, BLM-S and Act-D cause damage to the genetic material, but since mitotically dividing cells as cancer cells have a high metabolism rate, they show more DNA damage than non-dividing cells in response to these clastogenic agents which lead to the application of cancer therapy for BLM-S and Act-M.
The aim of the present study was to evaluate the induction of chromatid aberrations (CA) in two types of mouse- human hybridoma cells (i.e. HF2x653 and F3B6) by BLM-S and Act-D and to compare the frequency of various CAs with those induced in their parents, a human B-lymphocyte (WIL2.NS.6TG) and a myeloma mouse cell (NS-1) lines. Hybridoma cells are obtained from the fusion of two somatic cells with a similar or different origin which mostly have dissimilar chromosomal content in comparison to their parents. Therefore hybridoma cells have basically different nucleic acid content and consequently disparate cellular properties. This difference might be expressed in the amount of chromosome aberrations which are examined in this study. Various studies on the cytogenetic effects of BLM-S and Act-D in different cell systems [6-15] have already been conducted before but to the best of our knowledge this is the first cytogenetic study of BLM-S and Act-D on the hybridoma cells
2. Methods
Mutagens
Bleomycin sulfate (Nippon Kayaku Co) is commercially available as sterile lyophilized powder which has been diluted with normal saline and used at concentrations of 20 and 40 μg/ml in this study. Actinomycin D (Merck, Lyovac Cosmogenen) which is composed of 0.5 mg dactinomycin and 20mg mannitol was also diluted by normal saline and employed at concentrations of 0.1 and 1 μg/ml.
Cells
Four cell lines were used, two of which were heterohybridoma cell lines including HF2x653 and F3B6 produced from the fusion of mouse myeloma cells and human normal lymphocytes while the other two cell lines were non-hybrid cells. A human B-lymphocyte line, WIL2.NS.6TG, as human parent, and a myeloma mouse cell line, NS-1, as mouse parent were two non-hybrid cell lines. All the cells were provided by National Cell Bank of Iran, Pasture Institute, (Tehran, Iran).
Cell cultures and treatments
After defreezing, the cells were grown in RPMI 1640 medium (Sigma) supplemented with 10% inactivated fetal calf serum (Gibco BRL), 2mM of L-glutamine, 100 U/ml of penicillin (Sigma) and 100 μg/ml streptomycin (Sigma), and cultured at 37°C in a humidified atmosphere with 5% CO2. During the exponential growth period (about 106 cells/ml), the cultures were treated with the mentioned concentrations of Act-D and BLM-S for 3 hours separately. Colchicine was added at 4 μg/ml concentration during the last 1.5 h in all the cultures to arrest the cells in metaphase. Metaphase preparation was done according to the standard protocol (0.075 M KCl hypotonic shock, methanol:acetic acid 3:1 (Merck) fixation and air dried method for slide preparation). Finally, the chromosomes were stained in 10% Giemsa stain (Merck).
Cytogenetic analysis
Chromatid aberrations were analyzed in the first cycle metaphase after treatments. Only well spread metaphases were examined per treatment to determine and score the frequencies of chromatid aberrations. One hundred well spread metaphases were scored for each sample. The chromatid aberrations recorded were chromatid gaps, chromatid breaks (including acentric fragments, minutes and isochromatid breaks) and chromatid exchanges (including multiradials, di- or multicentrics and rings). We have followed according to the Hsu (1987) [16] criteria and disregard gaps or attenuated regions. Thus the sum of chromatid breaks and exchanges as ״total chromatid aberration ״ was used for statistical analysis.
Statistical analysis
Statistical comparisons were performed with the Kruskal-Wallis nonparametric and ANOVA with Duncan post hoc tests. The P value significance was set at 0.05. The data were analyzed by SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
Table 1 and Figure 1 show the frequency of various chromatid aberrations in the control groups and groups treated with BLM-S and Act-D at different concentrations.
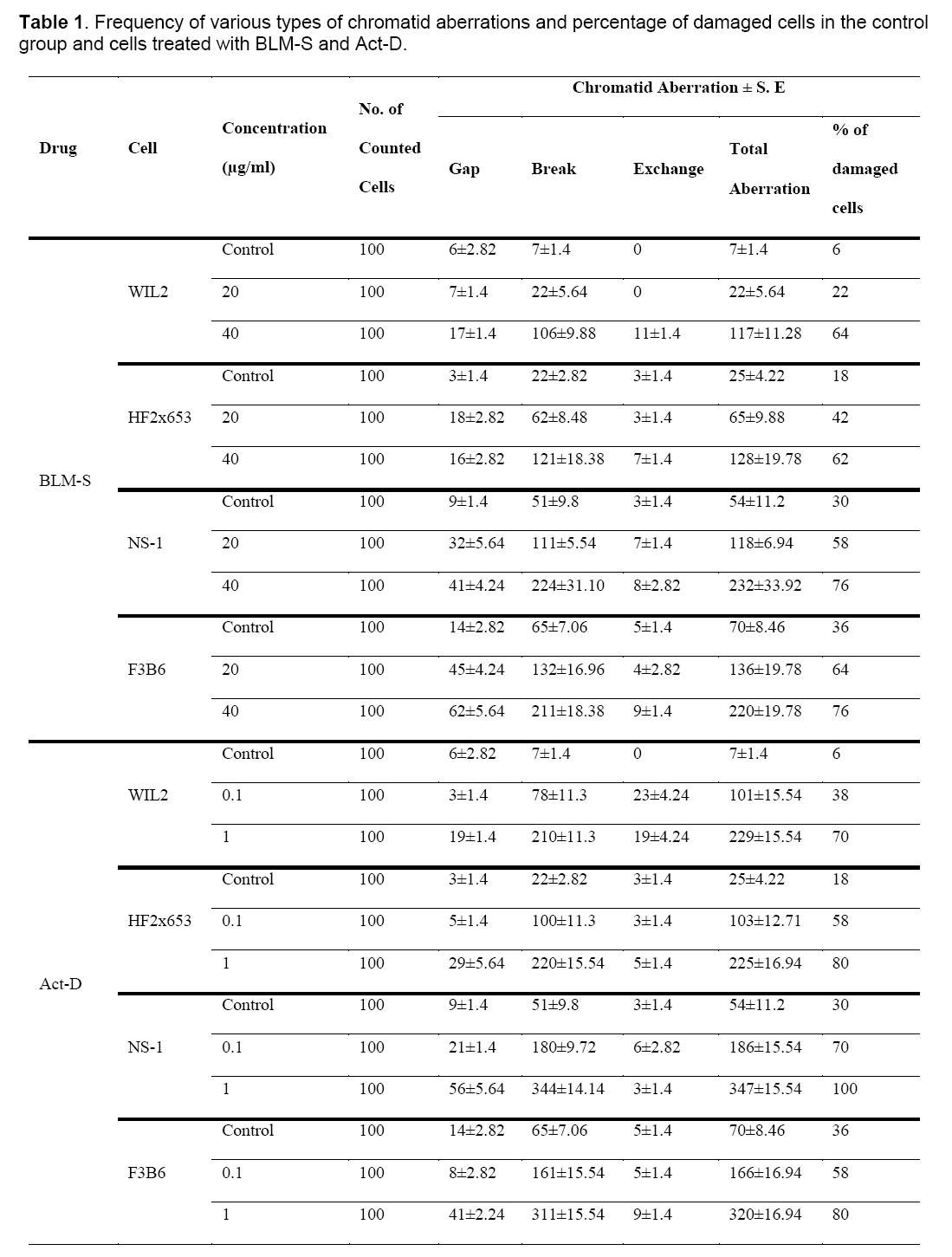
Figure 1: Column chart based on frequencies of various types of chromatid aberrations in different cell lines for the control group (a), BLM-S treatment at 20 μg/ml concentration (b), BLM-S treatment at 40 μg/ml concentration (c), Act-D treatment at 0.1 μg/ml concentration (d) and Act-D treatment at 1 μg/ml concentration (e).
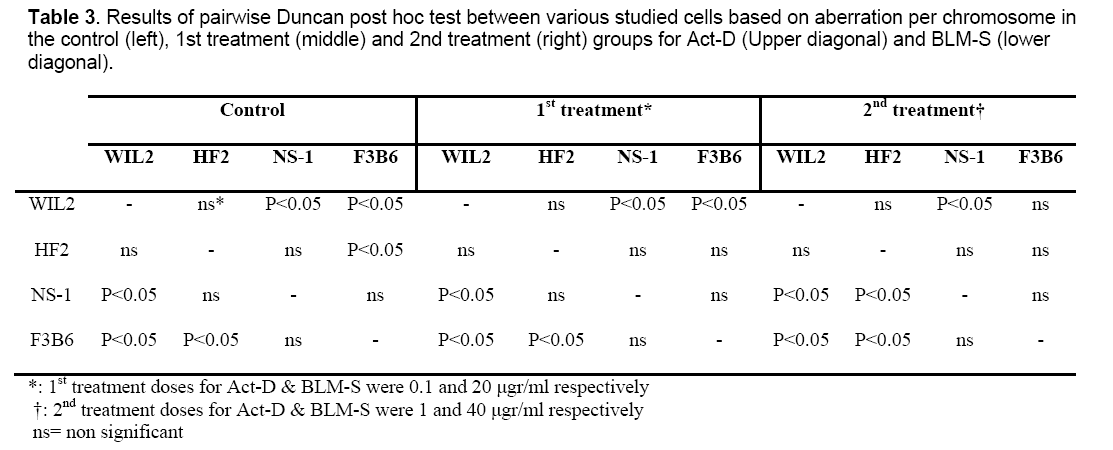
Since the studied cells in this research have different numbers of chromosomes, the analysis was done on the basis of aberration per chromosome which has been summarized in Table 2 for all the treated cell lines.
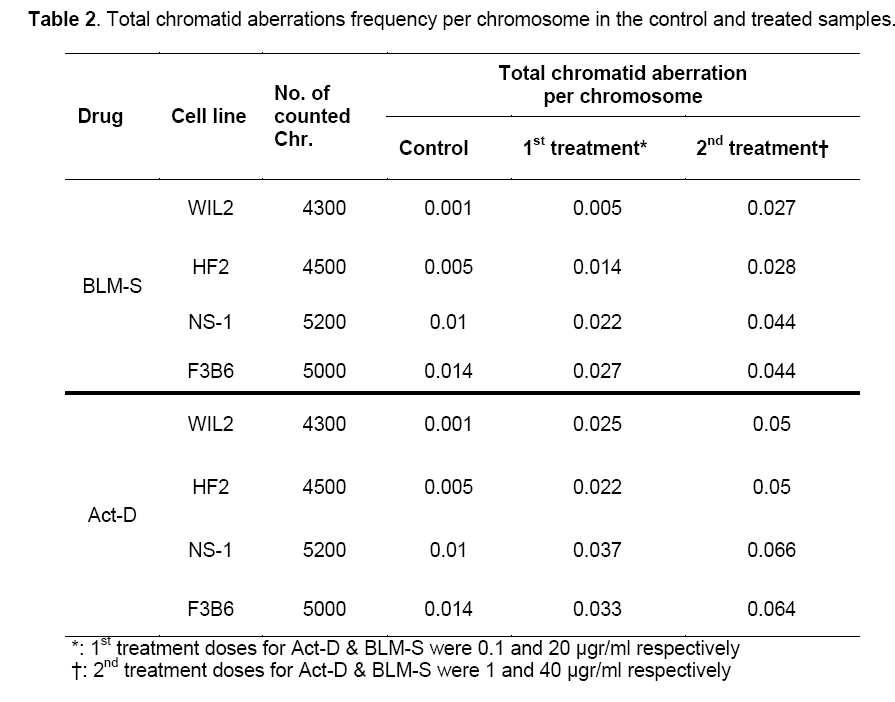
The results showed that in all cell treatments, both agents in both concentrations induce a significant increase of total chromatid aberrations compared to the control group (P<0.05). Additionally, treatments with higher doses (i.e. 40 μg/ml and 1 μg/ml for BLM-S and Act-D, respectively) in both agents differ significantly with the lower ones (i.e. 20 μg/ml and 0.1 μg/ml for BLM-S and Act-D, respectively) (P<0.05).
Results of pairwise Duncan post hoc test between the studied cells for both Act-D (Upper diagonal) and BLM-S (lower diagonal) treatments are also presented in Table 3 in a matrix format. Except for 1 μg/ml concentration of Act-D in which hybrid cells have not shown any significant difference in CA frequency with non-hybrid lines (P˃0.05) (Table 3, right, upper diagonal), in the rest of the applied concentrations, the responses of hybridoma cell lines differ significantly in comparison to non-hybrid lines. In the control group, HF2x653 hybrid cells do not show any significant difference with non-hybrid cell lines (NS1 and WIL2) while F3B6 cells differ significantly with WIL2 but not with NS-1 (Table 3, left). The same pattern is observed for 20 μg/ml dose of BLM-S (Table 3, middle, lower diagonal) but for higher concentrations of BLM-S (40 μg/ml), HF2x653 and F3B6 hybrid cell lines are located with WIL2 and NS-1 non-hybrid cells in the same category respectively (Table 3, right, lower diagonal). In the case of Act-D, HF2x653 cell line has no difference with non-hybrid cells in both concentrations. On the other hand, F3B6 shows a significant difference with only WIL2 cell line in both applied doses (Table 3, middle and right upper diagonal).
Hybridoma cells are produced from the fusion of two or more somatic cells which may originate from the same or different species. The whole number of hybrid cell’s chromosomes is not usually equal to the total number of parental chromosomes. After the fusion and in the early stages of cell growth all all parental chromosomes are present but in a few successive divisions some of them will be lost. When hybridoma cells are interspecies, chromosome loss occurs more frequently in the chromosomes of one of the parent cells that would cause the hybrid cell to be more similar to the other parent line [17]. Thus, nuclear environment of the hybridoma cells changes with chromosome loss which would probably alter the hybridoma cell specificities in comparison to the parental cells. The way of response to various types of chemotherapy drugs could be among these alterations. The present study was primarily performed to compare the clastogenic effects of BLM-S and Act-D on hybrid and non-hybrid cell lines.
BLM-S shows remarkable therapeutic efficacy for squamous cell carcinoma, malignant lymphoma and testicular carcinoma which with a radiomimetic mechanism exerts single and double strand breaks on DNA [2,18-20]. Various cell lines show differential sensitivity to BLM-S. However, it is not known if this difference is truly intrinsic or is caused by differential permeability of the cell membranes. BLM-S is a large hydrophobic compound and most likely unable to diffuse across cell membranes [20]. Although there is no consensus mechanism for BLM-S cell uptake, it has been proposed that BLM-S transferring across plasma membranes of Chinese hamster fibroblast and human head and neck carcinoma cell lines is mediating by binding to a receptor protein which leads it to the receptor mediated endocytosis pathway [21]. BLM-S resistance has been observed in those cells which had reduced BLM-S-membrane binding sites [22]. Moreover, lymphocytes with different BLM-S uptake rate show different responses to BLM-S treatment. Additionally, chromosome repair mechanisms have a major effect on the net chromatid aberration amount after drug treatment. Different and sometimes contradictory observations about the repairing ability of various cells have been reported.
DNA unwinding method in different cell types have revealed that most of the DNA breakage by bleomycin is repaired within 5 min of discontinuation of the treatment when slight repair of DNA nicks occurs if the bleomycin remains in the medium [23,24]. Pulsed field electrophoresis also showed a 50% rejoining of double strand breaks in Chinese hamster ovary wild type cells during 30 min after 1-h pulse bleomycin treatment [25] but G2 premature chromosome condensation technique did not measure the significant repair until 1-h after BLM-S removal [26].
Although 20 μg/ml treatment of BLM-S induced significant CA per chromosome in the treated cells compared to the control group, pairwise Duncan post hoc test matrix (Table 3) indicated that all the cell types were similar to the control group for 20 μg/ml dose of BLM-S treatment. On the other hand, for higher doses of BLM-S (40 μg/ml), hybrid cells had a completely different pattern of pairwise Duncan post hoc test. In other words, at 40 μg/ml of BLM-S treatment, HF2x653 and F3B6 hybrid lines responded exactly as their corresponding parents which were WIL2 and NS-1 respectively. Since repair ability or plasma membrane permeability of a hybrid cell as the other specificities of these cells are similar with their parent lines, this pattern of response is not unexpected. As mentioned previously, both concentrations of BLM-S treatment resulted in significant differences in the all studied cells, but it seems that higher doses of BLM-S are necessary to observe consistent reaction of hybrid lines as their corresponding parents. However this consistency somehow could be observable both in the control groups and lower dose treatments of BLM-S.
Act-D, marketed under the trade name Dactinomycin is one of the older chemotherapy drugs that has been successfully used in many cancer therapies such as gestational trophoblastic neoplasia, Wilms’ tumor, rhabdomyosarcoma and Ewing’s sarcoma [27-30]. Differential sensitivity of the cell lines also has been observed for actinomycin [31]. This differential sensitivity was also observed in our study relatively in the same way that had occurred for bleomycin treatment. In other words, cells responses are almost explainable by dividing them into two separate categories including HF2x653 and its corresponding parent line WIL2 versus F3B6, and its corresponding parent line NS-1. NS-1 is a myeloma cell line and has various types of genomic mutations and chromosomal damages including multiple translocations which could directly or indirectly involve those genes participating in cellular repair or drug resistance. Malfunction of these genes will ultimately increase the affected cell’s sensitivity to clastogens. As can be seen in Table 3, in all the treatments and even in the control cell lines, NS-1 cells always show a significant difference in aberration per chromosome frequency compared to the normal lymphocyte line, WIL2. This is more evident in higher concentrations of Act-D treatment (Table 3, right, upper diagonal) in which the only two differing cells are NS-1 and WIL2. Conversely, heterohybridoma lines responses usually are similar to one of their parents. In fact, careful assessments of metaphasic spreads of the studied cells confirmed that HF2x653 and F3B6 hybridoma cells have similar genomic backgrounds as WIL2 and NS-1 respectively whether in the chromosome content or type.
4. Conclusion
Briefly, the studied cell lines in this study have shown a variety of different responses to the applied treatments. WIL2 and NS-1 non-hybrid cells are situated in two opposite extremes while the hybridoma cells were seen in moderate positions of the mentioned spectrum but with a significant inclination to their corresponding parent lines.
Acknowledgements
This study was a part of a project supported by the Pasteur Institute of Iran. The authors wish to thank the personnel of National Cell Bank for their helps and Mrs. Ahmadi for her technical assistance.
References
- Harsch, A., Marzilli, L.A., Bunt, R.C., Stubbe, J. and Vouros, P. (2000) Accurate and rapid modeling of iron-bleomycin-induced DNA damage using tethered duplex oligonucleotides and electrospray ionization ion trap mass spectrometric analysis. Nucleic. Acid. Res, 28: 1978–1985.
- Povirk, L.F. and Austin, M. J. F. (1991) Genotoxicity of Bleomycin. Mutat. Res, 257: 127-143
- Sobell, H. (1985) Actinomycin and DNA transcription. Proc. Natl. Acad .Sci. USA, 82: 5328-5331.
- Waring, M. J. (1968) Drugs which affect the structure and function of DNA. Nature, 219: 1320-1325.
- Ostertag, W. and Kersten, W. (1965) The action of proflavin and actinomycin D in causing chromatid breakage in human cells. Exp. Cell Res, 39: 296-301.
- Bornstein, R.S., Hungerford, D.A., Hailer, G., Engstrom P.F. and Yarbo, J.W. (1971) Cytogenetic effects of bleomyein therapy in man. Cancer. Res, 31: 2004-2007.
- Hayez-Delatte, F., and Feremans W. (1975) Etude des aberrations chromosomiques provoqudes par la bleomycin sur les lymphocytes humalns in vitro, Bull. Cancer, 62: 29-36.
- Hittelman, W.N., and Rao P.N. (1974) Bleomycin induced damage in prematurely condensed chromosomes and its relationship to cell cycle progression in CHO cells. Cancer. Res, 34: 3433-3439.
- Schinzel, A., and Schmid, W. (1976) Lymphocyte chromosome studies in humans exposed to chemical mutagens. The validity of the method in 67 patients under cytostatic therapy. Mutat. Res, 40: 139-166.
- Tamura, H., Sugiyama, Y., and Sugahara, T. (1974) Effect of bleomycin on the chromosomes of human lymphocytes at various cell phases. Gann, 65: 103-107.
- Datta, S., Mallick, P. and Bukhsh, A.R. (1999) Efficacy of a potentized homoeopathic drug (Arsenicum Album-30) in reducing genotoxic effects produced by arsenic trioxide in mice: II. Comparative efficacy of an antibiotic, actinomycin D alone and in combination with either of two microdoses. Complement. Ther. Med, 7:156-163.
- Pathak, S., McGill, M. and Hsu, T.C. (1975) Actinomycin D effects on mitosis and chromosomes: sticky chromatids and localized lesions. Chromosoma, 50:79-88
- Chakrabarti, J., Biswas, S.J. and Khuda-Bukhsh, A.R. (2001) Cytogenetical effects of sonication in mice and their modulations by actinomycin D and a homeopathic drug, Arnica 30. Indian. J. Exp. Biol, 39:1235-1242
- Mozdarani, H. and Saberi A.H. (1994) Induction of cytogenetic adaptive response of bone marrow cells to radiation by therapeutic doses of bleomycin sulfate and actionmycin D as assayed by the micronucleus test. Cancer. Lett, 78: 141-150
- Mozdarani, H. and Jadidi, M. (1995) The interaction of radiation and intercalating agents in normal bone marrow cells as evaluated by spleen colony assay technique: The effects of bleomycin sulfate and actionmycin D. Med. J. I.R. Iran, 9: 137-145.
- Hsu, T.C. (1987) Genetic predisposition to cancer with special reference to mutagen sensitivity, In Vitro Cell. Dev. Biol, 23: 591-603.
- Onishi, T., Berglund, C. and Reeder R.H. (1984) On the mechanism of nucleolar dominance in mouse- human somatic cell hybrids. Proc.Nati.Acad.Sci.USA, 81:484-494
- Kuo, T.M. and Hsu T.C (1978) Biochemical and cytological studies of bleomycin action on chromatin and chromosome. Chromosoma, 68: 229-240
- Povirk, L.F. (2006) Biochemical mechanisms of chromosomal translocations resulting from DNA double strand breaks. DNA. Rep. (Amst), 5: 1199-1212.
- Chen, J. and Stubbe, J. (2005) Bleomycin: towards better therapeutics. Nat. Rev. Cancer, 5: 102-112.
- Pron, G., Belehradek, J.Jr. and Mir, L.M. (1993) Identification of a plasma membrane protein that specifically binds bleomycin. Biochem. Biophys. Res. Commun, 194: 333–337.
- Pron, G., Mahrour, N., Orlowski, S., Tounekti, O., Poddevin, B., Belehradek, Jr. J. and Mir, L.M. (1999) Internalisation of the bleomycin molecules responsible
- for bleomycin toxicity: a receptor-mediated endocytosis mechanism. Biochem. Pharmacol, 57: 45–56.
- Bianchi, N.O. and Lopez-Larraza D.M. (1991) DNA damage and repair induced by bleomycin in mammalian and insect cells. Environ. Mol. Mutagen, 17: 63-68.
- Lopez-Larraza, D., De Luca J.C. and Bianchi N.O. (1990) The kinetics of DNA damage by bleomycin in mammalian cells. Mutat. Res, 232: 57-61.
- Giaccia, A.J., Lewis, A.D., Denko, N.C., Cholon, A., Evans, J.W., Waldren, C.A., Stamato T. and Brown J.M. (1991) The hypersensitivity of the Chinese hamster ovary variant BL-10 to bleomycin killing is due to a lack of glutathione S-transferase-a activity. Cancer. Res, 51: 4463-4469.
- Sognier, M.A., Hittelman, W.N. and Rao, P.N. (1979) Effect of DNA repair inhibitors on the induction and repair of bleomycin-induced chromosome damage. Mutat. Res, 60:61-72.
- Cole, K. E., Thomas, W.J, Michael, M.L, Benjamin, F.T and Ih-Chang, H. (1989) Comparative effects of three carcinogens on human, rat and mouse hepatocytes. Carcinogenesis, 10: 139-143
- Turan, T., Karacay, O., Tulunay, G., Boran, N., Koc, S., Bozok, S. and Kose, M. (2006) Results with EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) chemotherapy in gestational trophoblastic neoplasia. Int. J. Gynecol. Cancer, 16: 1432–1438.
- Abd El-Aal, H., Habib, E. and Mishrif, M. (2005) Wilms' Tumor: The Experience of the Pediatric Unit of Kasr El-Aini Center of Radiation Oncology and Nuclear Medicine (NEMROCK). J. Egypt. Natl. Canc. Inst, 17: 308–311.
- Khatua, S., Nair, C. and Ghosh, K. (2004) Immune-mediated thrombocytopenia following dactinomycin therapy in a child with alveolar rhabdomyosarcoma: the unresolved issues. J. Pediatr. Hematol. Oncol, 26: 777–779.
- Vig, B.K. (1977) Genetic toxiology of mitomycin C, actinomycins, daunomyein and adriamycin. Mutat. Res, 49: 189-238.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences