Biological Control of Damping-Off and Root-Rot Diseases
Nebia Bouzidi, Khalladi Mederbal
1 Research Laboratory Geo-environment and Development, University of Mascara, Algeria
2 University of Tiaret, Algeria.
- Corresponding Author:
- Nebia Bouzidi
Research Laboratory Geo-environment and Development
University of Mascara, Algeria
Tel: 0791182816
E-mail: lina_kholoud@yahoo.fr
Received date: December 14, 2015; Accepted date: January 30, 2016; Published date: January 31, 2016
Citation: Bouzidi N, Mederbal K, Biological Control of Damping-Off and Root-Rot Diseases. Electronic J Biol, 12:1
Abstract
Biological control of plant diseases has been considered a viable alternative method to manage plant diseases. Biocontrol is environmentally safe and in some cases is the only option available to protect plants against pathogens. Through our study, we tried to find out the possibility of using the essential oil of Artemisia herba alba as a biological control agent. The antifungal activity of the oil was determined and it was found that this essential oil have an interesting effect on conidia germination, mycelia growth, and the sporulation of Fusarium oxysporum f. sp. ciceris (Chickpea Fusarium Wilt), Fusarium solani (Chickpea root-rot) and Globisporangium ultimum (Aleppo pine damping off and root-rot diseases). Results obtained highlight the importance of this subject as it can offer the possibility of using plant derivatives in disease management against these soil-borne pathogens
Keywords
Aleppo pine; Chickpea; Essential oil; Fungal disease.
1.Introduction
Plant diseases are mostly controlled by chemical pesticides and in some cases by cultural practices. However, the widespread use of chemicals in agriculture has been a subject of public concern and scrutiny due to the potential harmful effects on the environment, their undesirable effects on non-target organisms and possible carcinogenicity of some chemicals [1]. Other problems include development of resistant races of pathogens, a gradual elimination and phasing out of some available pesticides and the reluctance of some chemical companies to test new pesticides due to the problems with registration process and cost. The need for the development of non-chemical alternative methods to control plant diseases is therefore clear [1]. Biological control of plant diseases has been considered a viable alternative method to manage plant diseases. Bio control is environmentally safe and in some cases is the only option available to protect plants against pathogens.
Chickpea Fusarium wilt and Chickpea root-rot caused, respectively, by Fusarium oxysporum f. sp. ciceris (FOC) and Fusarium solani (FS), represented the most widespread and destructive diseases of this legume crop. It is considered a limiting factor to Chickpea production in Algeria causing significant economic losses [2]. Globisporangium ultimum (Trow) Uzuhashi, Tojo and Kakish (syn. Pythium ultimum Trow, syn. P. ultimum Trow var. ultimum). GU is a known oomycetal species from Pythiums causing damping-off and root-rot on Aleppo pine (Pinus halepensis Mill.). This disease occurred under cool conditions, and Aleppo pines were significantly affected, reducing seedling emergence [3].
For controlling these diseases, cultural practices and the use of other methods are the most common strategies. However, they are either not available or effective. Biological control methods using antagonistic microorganisms [4] or plant derivatives [5,6] offer a powerful and eco-friendly alternative to the use of synthetic chemicals that have been applied to control plant diseases.
Artemisia herba-alba Asso (or the white wormwood) known in Arabic as “Chih” is a very abundant steppe plant on the highlands but rarefying in the septentrional Sahara, the central Sahara and at altitude above 1400 m (in the Hoggar) [7]. This plant interests more than one sector. The pastoralism of the large paths finds there an irreplaceable field and its essential oil is intended primarily for the industry of perfumery. Medicinal and aromatic plants and their essences have several biological activities. In phytotherapy, they are used for their antiseptic properties against the infectious diseases of bacteria. In the pharmaceutical preparations, phenolic terpenes, like thymol and carvacrol, are often used as antimicrobic and antifungic disinfectants. In the phytosanitary and agro-alimentary fields, essential oils or their active substances could be employed like agents of protection against fungi and microorganisms who invade the foodstuffs.
The use of plant extracts has long been recognized as an area of investigation [8] against various fungal diseases. The present work aimed to evaluate the antifungal of essential oil of Artemisia herba alba Asso.
2. Materials and Method
2.1 Fungal isolates
The fungal support used to determine the in-vitro antifungal activity consists of three fungal strains: Fusarium oxysporum (FOC), Fusarium solani (FS) and Globisporangium ultimum (GU). It was provided by the laboratory of Plant Pathology, University of Mascara.
2.2 Antifungal activity
Mycelia growth: Fungal isolates are cultivated on PDA medium during seven days at 25°C in darkness [9,10]. The choice of the concentrations of the essential oil is carried out on the basis of preliminary test. The mycelia growth was carried out by direct contact method, which consists in adding essential oil in PDA medium at 45°C to obtain concentrations of 1000, 500 and 250 ppm. After obtaining these various dilutions and their solidification in Petri dishes (15 ml/ dish), mycelia discs of 1 cm of diameter, issue of 7 days old culture of each isolate, was deposited in the center of each Petri plate. Control plates containing the culture medium and the mycelia disc of each isolate without essential oil were realized. Each test is repeated three times. After incubation at 25°C during 7 days, by taking account of the growth of control, the percentage of inhibition (PI) is calculated by the following formula:
PI %= (dT-dE) /dT × 100;
PI %: Percentage of Inhibition; dT: Average diameter of the growth zone of the control; dE: Average diameter of the growth zone of the test.
Evaluation of germination: The collected suspension of the spores is adjusted at a rate of 105 spores/ml of distilled water using a cell of Malassez. We spread out 0.1 ml of this suspension on Petri dishes containing PDA medium with essential oil at the same concentrations previously mentioned. Three repetitions are carried out simultaneously for each concentration. The counting of the spores germinated or not was carried out on a total of 200 spores after 5 to 18 hours of incubation at 25°C in darkness. A spore is considered germinated if the length of the germinatif tube is higher than its smaller diameter. Percentage of inhibition of the germination of the spores Ig is given according to the following formula:
Ig= (N0-Nc) /N0 × 100%
Where N0: The number of the spores having germinated in the culture medium without essential oil (control); Nc: The number of the spores germinated in the presence of a concentration (C) of essential oil [10].
Evaluation of the sporulation: The evaluation of the sporulation was estimated from plates having been used to study the mycelia growth. Four discs of 5 mm in diameter were cut out with a perforating punch (cookie cutter) along the diameter of the same colony and were put in a tube containing 1 ml of distilled water. The spores suspension is then agitated using a vortex in order to release the spores of the conidiophores. These experiments are repeated three times. After counting the full number of the spores using a cell of Malassez at a rate of 10 counting by suspension, the values are expressed of number of the spores per unit of area (mm2). The percentage of inhibition of the sporulation (IS) is calculated by the following formula:
IS %= [(N0–Nc) /N0] × 100
Where N0: The mean number of the spores estimated for the control and Nc: The mean number of the spores estimated in the presence of essential oil [10].
3. Results and Discussion
3.1 Antifungal activity
The difficulty of fighting against root diseases by synthetic pesticides explains the misuse of these pesticides by producers. Therefore, this misuse of pesticides poses a growing problem of profitability, risk of residues of synthetic products in plants and environmental pollution [11].
Given the concern about the growth in the use of synthetic pesticides and the effect of their residues on consumer health, several studies have focused on the use of plant extracts to develop new fungicides to fight against fungal diseases of plants.
This study aims to evaluate the antifungal activity of the essential oil extracted from "Artemisia herba alba" against three pathogenic Fusarium oxysporum f ciceris (FOC), Fusarium solani (FS) and Globisporangium ultimum.
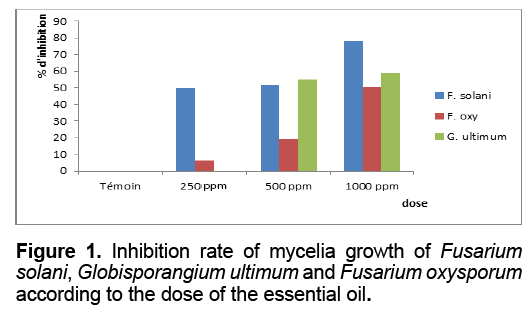
Mycelia growth: The antifungal activity of essential oil was tested by the method of diffusion and the appreciation of the diameters of the inhibition zones in vitro. Obtained results of the direct contacts method show that the mycelial growth of the various strains is affected by the various concentrations of essential oil. Indeed, more the concentration of essential oil increases more the rate of inhibition increases. A maximum of effect is obtained with the amount of 1000 ppm, this oil reduced the development of Fusarium solani, Globisporangium ultimum and Fusarium oxysporum with a rate of 78.33, 58.88 and 50.61% respectively (Figure 1).
We also note that there are differences in sensitivity between species belonging to the same genus (Fusarium oxysporum and Fusarium solani). By comparing the behavior of the species and strains for each dose of differences in susceptibility or resistance are noted. The difference in effect between doses is highly significant on all three strains tested. Fusarium oxysporum and Globisporangium ultimum behave in the same way at the dose of 250 ppm, but Fusarium solani is most sensitive at this dose. Observing the averaged results of the effect of 500 ppm, one can say that Fusarium oxysporum is more resistant than the other strains with only 19.14% of growth inhibition. For the dose of 1000 ppm, Fusarium solani is very sensitive with 78.33% inhibition, whereas Globisporangium ultimum and Fusarium oxysporum have the same sensitivity threshold at this dose.
The mechanisms of action of essential oils whereby the mycelial growth can be reduced or completely inhibited have been proposed. So, Lucini et al. [12] and El Badawy [13] reported that the essential oils act upon the hyphae of the mycelium, which causes the output of the components of cytoplasm, loss of stiffness and loss of integrity of the cell wall of hyphae, causing its collapse and death of the mycelium.
Evaluation of germination: For the germination of the spores, it appears that the most important effect was observed for Globisporangium ultimum. An inhibiting activity of 100% was noticed by the application of the two concentrations (500 ppm and 1000 ppm) on the germination of Globisporangium ultimum, on the other hand the percentage of germination at the concentration of 250 ppm is lower than the value of CI50. On the basis of percentage of germination of the conidia, essential oil appeared to be active on all strains tested and at all the concentration of essential oil used. Analysis of variance and LSD test of Scheff shows that there is a highly significant difference between the dose of 250 ppm and both doses of 500 to 1000 ppm on the behavior of Globisporangium ultimum. All strains have the same sensitivity to dose 250 ppm.
Globisporangium sp is widely studied because of its economic importance. Indeed, it can cause severe damage on plants; it is considered a formidable pest of forest crops. As with other treatments, Lazrag analyzed the essential oil antifungal capacity of inule "Inula viscosa L." Bacillus subtillis and Trichoderma atroviride biocontrol agents and a chemical fungicide "Benomyl" against Globisporangium sp. The results showed that these treatments used did affect neither conidial germination nor mycelia growth of this species. However, our results showed a strong antifungal activity of the essential oil of A. herba alba against Globisporangium sp. so can say with some caution that our plant, and through the use of its essential oil, can be a very effective solution, and so expected to fight against this ferocious and chronic parasite (Figures 2-4).
Evaluation of the sporulation: The three percentages of sporulation of each studied strain are higher than CI50 (FOC, 58.53%;GU, 63.46% and FS, 75.31%).For the low concentration (250 ppm), we find that the percentage of inhibition of FOC and FS is more or less equal to CI50, contrary in GU which has a percentage lower than the value CI50. Therefore, this essential oil has an inhibiting effect on the sporulation of the strains studied with concentrations of 500 and 1000 ppm
According to the statistical analysis, we find that all doses were significantly different in their effect on sporulation Globisporangium ultimum. Following the results (below), we may infer that the essential oil of sagebrush has a broad spectrum of action on the tested fungi. It inhibited mycelial growth, germination and sporulation in F. oxysporum, F. solani and Globisporangium ultimum. Its antifungal activity is primarily a function of its composition.
Several studies have demonstrated the efficacy of antifungal monoterpenes [14-16]. The antifungal activity of A. herba alba essential oil in this study can be attributed to the presence of high percentages of oxygenated monoterpenes. However, other minor compounds in the oil tested such as: terpinene-4-ol can lead to an interesting antifungal activity [17-20]. The antifungal activity decreases depending on the chemical functions:
Hydrocarbons?Ethers?Ketones?Aldehydes?Alcohol s?Phenols [21].
4. Conclusion
The medicinal and aromatic plants constitute an important and inexhaustible source of substance and natural bioactive compounds. By the means of this work, we tried to determine the antifungal capacity of the essential oil of the white wormwood “Artemisia herba alba Asso”, and its effect against two fungi responsible for the damping-off and root-rot disease. The antifungal activity was tested by the method of direct contact and the evaluation of the sporulation and germination. The results showed that examined oil has an important antifungal activity against the strains tested [22,23].
References
- Cook R, Baker KF. (1983). The nature and practice of biological control of plant pathogens.
- Labdi M. (1990). Chickpea in Algeria. Options Méditerranéennes, SérieSéminaires. 9: 137-140.
- Lazreg F, Belabid L, Sanchez J, et al. (2013). First report of Globisporangium ultimum causing Pythium damping-off on Aleppo pine in Algeria, Africa and the Mediterranean Region. Plant Disease. 97: 1111.
- Zaim S. Belabid L, Bellahcene M. (2013). Biocontrol of chickpea Fusarium wilt by Bacillus spp. rhizobacteria. Journal of Plant Protection Research. 53: 177-183.
- Belabid L, Si-moussaL, Bayaa B. (2010). Effect of some plant extracts on the population of Fusarium oxysporum f. sp. lentis, the causal organism of lentil wilt. Advanced Environmental Biology. 4: 95-100.
- Moussa SI, Belabid LL, Tadjeddine A, Bellahcene M, Bayaa B. (2010). Effect of some botanical extracts on Fusarium oxysporum f. sp. albedinis, the causal agent of Bayoud disease in Algeria. Arab Journal of Plant Protection. 28: 71-79.
- Ozenda P. (1988). Flora of the Sahara. Edits. CNRS. 141.
- Sahayaraj K, Namasivayam SKR, Borgio JAF. (2006). Influence of three plants extracts on Fusarium oxysporum f. sp. ciceris mycelium growth. Journal of Plant Protection Research. 46: 335-338.
- Dohou N, Yammi K, Badoc A, Douira A. (2004). Activité antifongique d’extraits de Thymelaea lythroides sur trois champignons pathogènes du riz. Bull Soc Pharm Bordeaux. 143: 31-38.
- Khaladi A, Meddah B, Moussaoui A, Benmehdi H, Gouri S. (2012). Screening phytochimique et effet antifongique de certains extraits de plantes sur le développement in vitro des moisissures. European Journal of Scientific Research. 80: 311-321.
- Doumbouya M, Abo K, Lepengue AN. (2012). Activités comparées in vitro de deux fongicides de synthèse et de deux huiles essentielles, sur des champignons telluriques des cultures maraîchères en Côté d’Ivoire. J Appl Biosciences. 50: 3520-3532.
- Lucini EI, Zunino MP, Zygadlo JA. (2006). Effect of monoterpenes on lipid composition and sclerotial development of Sclerotium cepivorum Berk. Journal of phytopathology. 154: 441-446.
- El Badawy MEI, Abdelgaleil SAM. (2014). Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Industrial crops and products. 52: 776-782.
- Chebli B, Achouri M, Idrissi Hassani M, Hmamouchi M. (2003). Antifungal activity of essential oils from several medicinal plants against four postharvest Citrus pathogens. Phytopathologia Mediterranea 42: 251-256.
- Salamci E, Kordali S, Kotan R, Carin A, Kaya Y. (2007). Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum Var. chiliophyllum. Biochemical systematic and ecology. 35: 569-581.
- Kordali S, Kotan R, Mavi A. (2005). Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum and Artemisia spicigera essential oils. Journal Agricultural Food and Chemistry. 53: 9452- 9458.
- Wang SY, Chen PF, Chang ST. (2005). Antifungal activities of essentials oil and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bio ressource Technology. 96: 813-818.
- Dorman HJD, Deans SG. (2000). Antimicrobial agents from plants: antibacterial activity of plant volatile oils. Journal of Applied Microbiology. 88: 308-316.
- Hu H, Zheng X, Hu H. (2012). Chemical composition, antimicrobial, antioxidant and cytotoxic activities of the essential oil from the leaves of Acant hopanax leucorrhizus (oliv.) Harms. Environnemental toxicology and pharmacology. 34: 618-623.
- Jordan JM, Lax V, Rota MC, Loran S, Sotomayor JA. (2013). Effect of bioclimatic area on the essential oil composition and antibacterial activity of Rosmarinus officinalis L. Food control. 30: 436-468.
- Hernandez Ochoa LR. (2005). Substitution de solvants et matières actives de synthèse par un combine « solavnat/actif » d’origine végétale. Thèse de doctorat. Institut national polytechnique de Toulouse.
- Lazreg F. (2014). Importance de fonte de semis du pin d’Alep « Pinus halepensis Mill.) dans le Nord –Ouest Algérien: Identification morphologique et moléculaire des espèces du genre Fusarium et globisporangium, pouvoir pathogène et moyens de lutte. Thèse de Doctorat. Université de Tlemcen.
- Almadi F, Sadeghi S, Madarresi M, Abiri R, Mikacli A. (2010). Chemical composition, in vitro, antimicrobial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth of Iran. Food and chemical toxicology. 48: 1137-1144.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences