Assessment of Internal Absorbed Dose in the Human Abdominal Organs from Two Renal Radiopharmaceuticals Based on Experimental Mouse Data
BentolHoda Mohammadi, Seyed Pezhman Shirmardi*, Mostafa Erfani, AA Shokri
BentolHoda Mohammadi1, Seyed Pezhman Shirmardi2,*, Mostafa Erfani2, AA Shokri1
1Department of Physics, Payame Noor University,Tehran, Iran
2School of Radiation Application Research, Nuclear Science and Technology, Research Institute (NSTRI), Tehran, Iran
- *Corresponding Author:
- Seyed Pezhman Shirmardi
School of Radiation Application Research
Nuclear Science and Technology, Research Institute (NSTRI)
Tehran, Iran
Tel: 00989167398416
E-mail: p_shirmardi@aut.ac.ir
Received Date: April 18, 2018; Accepted Date: June 03, 2019; Published Date: June 10, 2019
Citation: Mohammadi B, Shirmardi SP, Erfani M, et al. Assessment of Internal Absorbed Dose in the Human Abdominal Organs from Two Renal Radiopharmaceuticals Based on Experimental Mouse Data. Electronic J Biol,15:2
Abstract
Background: Radiopharmaceuticals have several applications in medicine such as imaging and therapeutic processes. Radiopharmaceutical renal scintigraphy presents important functional data to assist in the diagnosis and management of patients.
Methods and findings: In this research, the human effective dose from two important renal imaging agents (99mTc- MAG3 and 131I-Hippuran) is estimated, after injection of those in animal body. Effective dose in human body is estimated using MIRD method and MCNP simulation code (with F6 and *F8 tally) for different organs. Sources with energies of 140keV (gamma) for 99mTc and 364 keV (gamma), 192 keV (average energy of beta) for 131I are considered. The results of 99mTc-MAG3 and 131I-Hippuran dosimetry showed that the lung and stomach (for 99mTc-MAG3) and stomach and lung (for 131I-Hippuran) had the most effective dose relative to the other organs respectively.
Conclusion: There is no good agreement between mouse model and Stabin’s human model for 131I-Hippuran.
Keywords
MCNPX simulation code; MIRD method; 99mTc- MAG3; 131I-Hippuran; Effective dose.
1. Introduction
Radiopharmaceuticals are applied in nuclear medicine for therapy and diagnosis of disease and organ function. The radiopharmaceuticals can enter the human body as radioisotopes alone or radioisotopes connected to different chemical compounds. Some radiopharmaceuticals are used for renal function and anatomy. The radiopharmaceuticals for investigation of renal function and structure can be divided into three categories: first, the radiopharmaceuticals filtered by the glomerulus, second, the radiopharmaceuticals retained in the renal tubules via proximal tubule receptor-mediated endocytosis from the glomerular filtrate, and third, the radiopharmaceuticals primarily secreted by the renal tubules via the organic anion transporter [1]. 131I-Hippuran and 99mTc-MAG3 are two important radiopharmaceuticals in renal scintigraphy. 99mTc-MAG3 is a renal tubular agent which was introduced in 1986 as an alternative for the use of 131I-Hippuran with similar pharmacokinetic and human renogram pattern. 99mTc-MAG3 is highly protein-bound and is removed from the plasma primarily by the organic anion transporter 1 [2]. 99mTc-MAG3 is used more than 99mTc-DTPA for patients with suspected obstruction and impaired renal function (IRF) and is applied in almost 70% of the renal scans performed in the US [1,3,4].
131I-iodohippurane is a radiopharmaceutical used in diagnostics of kidneys malfunction and renal tract obstructions. After injection, 131I-iodohippurane is excreted by the renal system rapidly. The maximum uptake in renal system occurs almost within 2 to 5 minutes of injection. The peak of uptake often depends on the nature of the kidneys diseases, the patient hydration and extent of renal impairment. More than 60% of the mentioned radiopharmaceutical binds reversibly with plasma proteins. The renal excretion for this compound is mainly by tubular secretion (80%) and glomerular filtration (20%). The clearances of these renal radiopharmaceuticals are often characterized as the effective renal plasma flow. 131I-Hippuran is cleared from plasma by tubular secretion. However,131I is far from optimal with regard to physical properties and hence to image quality and radiation burden. Preliminary studies of 99mTc-MAG3 in normal volunteers indicated that plasma clearance was less than that of 131I-Hippuran [5]. 131I-Hippuran has the poor imaging characteristics and deliver a high radiation dose. The study by Erbslöh- Möller et al. [6], demonstrated a better sensitivity of 131I-hippuran renography than 99mTc-DTPA scintigraphy to diagnose renovascular hypertension (RVH). Although the clearance of 99mTc-MAG3 is only 50%–60% that of OIH (131I-Hippuran), the 99mTc-MAG3 clearance is highly correlated with the clearance of OIH, and the 99mTc- MAG3 clearance can be used as an independent measure of renal function [7,8].
The body receives radiation dose from the radiopharmaceuticals. Appraising the internal radiation dosimetry of radiopharmaceuticals is an essential part of the usage and development of novel radiopharmaceuticals. Many studies have been completed for calculation of the internal absorbed dose after injection of radiopharmaceuticals in body [9-15]. In 2004, Angela Keleher et al. calculated the dose of 92.5MBq of 99mTc - sulfur colloid on pregnant women, the highest dose calculated to the fetus was 7.74E-2mGy [16]. In 2012, Shahbazi et al. determined the absorbed dose of different organs resulting from 99mTc-dioxide phosphene where the highest dose of 38.73 E-4mGy/MBq was delivered to the kidney [14]. In 2014, the authors have recently calculated and compared effective dose of different organs of body resulting from injection of two radiopharmaceuticals of 99mTc-Bombesin and 67Ga-Bombesin [15].
There are several methods to calculate the internal absorbed dose of radiopharmaceuticals. One of the methods is “Medical internal radiation dose (MIRD)”. MIRD has offered a method for calculation of gamma beam energy on organs and tissues. This method is based on absorbed fraction; it means that a fraction of emitted energy from source organs is absorbed on target organs [9]. MCNP code is a second method for calculating internal dose of body. MCNP is a generalpurpose Monte Carlo N-Particle code that can be used for some particles transport. Specific areas of application include, but are not limited to, radiation protection and dosimetry, radiation shielding, radiography, medical physics etc. The code treats an arbitrary three-dimensional configuration of materials in geometric cells bounded by first-and seconddegree surfaces and fourth-degree elliptical tori [17].
The purpose of this research is the calculation and comparison of human internal absorbed dose of two renal radiopharmaceuticals (99mTc- MAG3 and 131I-Hippuran) resulting from 1 MBq after intravenous injection to mice.
Materials and Methods
99mTc was prepared from the decay of 99Mo on an aluminum oxide column in a bench-top generator. The 99mTc was eluted from the generator in sterile NaCl (0.9% w/v) at room temperature in the form of sodium pertechnetate solution. Internal dosimetry calculations were done based on the 140 keV peak for 99mTc and 364 keV (gamma), 192 keV (average energy of beta) for 131I.
Absorbed dose on organs in overall is defined by following relation:
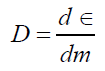 (1)
(1)
d ε : Average energy of ionizing radiation for dm.
By considering the type of particle on creating biologic effects, equivalent dose is applied, which is equal to absorbed dose multiplied by the radiation weighting factor.
H=DW (2)
The effective dose is calculated by multiplying the equivalent dose (H) by a tissue weighting factor (WT). Effective dose, is a quantity that in addition to considering role of different beams on emergence of biologic effects, also considers the effect of taking beams by various tissues and it is equal to multiply of equivalent dose in tissue weighting factor [18,19].
E=WT*H (3)
After preparation and injection of radiopharmaceuticals, animal studies were carried out in accordance with the UK Biological Council’s Guidelines on the Use of Living Animals in Scientific Investigations. The activity was measured with well-type counters before and after administration of the radiopharmaceutical. After injecting radiopharmaceutical to mice, they were sacrificed and their organs dissected. Three samples, from each organ, were dissected, weighed and then counted to determine the percentage of injected dose per gram (which was equivalent to the percentage of injected activity per gram %IA/g: %ID/g); all the organ activity measurements were normalized to injected activity.
Activity concentration of radiopharmaceuticals at times (t) was calculated as the percentage of injected activity of each of organs [15,20,21].
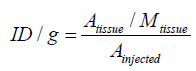 (4)
(4)
Where A tissue is the concentrated activity on sample tissue, M tissue is mass of sample tissue and A injected is total injected activity to mice.
After that, the activity accumulation in source organs is calculated by following method.
2.1 Cumulated radioactivity and dose calculation
The dose of radiation delivered from a source tissue to a target tissue is dependent on the value of radioactivity in the source tissue and the time length of the radiopharmaceuticals resides in the source tissue. Due to the continuous uptake and elimination of the radiopharmaceutical administered to the living system, and physical decay of the radionuclide, the radioactivity in each organ is dependent on time (Function of time). The cumulated radioactivity, Ã, can~ be shown as eqn. (5):
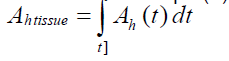 (5)
(5)
Ah (t): activity of each tissue on time (t). A᷉ accounts for the radioactivity in the tissue, and how long it resided in source tissue.
After calculation the activity for organs at different times, related activity curves were drawn for them. Then, using the curve-fitting method, cumulated radioactivity values were obtained.
For converting accumulated activity of mice organs to human organs, Sparks and Idogan method is used which is shown in eqn. (6) [6,15]:
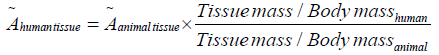 (6)
(6)
Due to the length and attenuation between the source tissues and target tissues, just a fraction of the energy emitted by the source tissue is absorbed by the target organ. This energy fraction needs to be quantified so that the total absorbed dose by the target tissue can be estimated.
The equation for absorbed dose in the MIRD system is shown as eqn. (7)
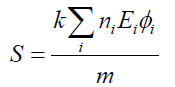 (7)
(7)
Where ni is the number of radiations with energy E emitted per nuclear transition, Ei is the energy per radiation (MeV), φi is the fraction of energy absorbed in the target, m is the mass of target region (g or kg) and k is proportionality constant (rad-g/μCi-hr-MeV or Gy-kg/MBq-sec-MeV).
Absorbed dose was calculated by using MIRD formula [15];
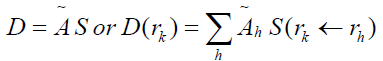 (8)
(8)
Where D(rk) is absorbed dose of the target tissue (rad or Gy), ̃ℎ is the accumulated activity in source tissue (μCi-hr or MBq-sec) and S (rk ←rh ) called S factor which is defined as the mean absorbed dose to the target region rk per unit accumulated activity in the source region rh. The S factor represents the physical decay characteristics of the radioisotopes, the range of the emitted radiations, and the organ size. The S factors have been taken from the tables presented in the medical internal radiation dose No. 11 [12] and also available in https://doseinfo-radar. com/RADARphan.html. Figure 1 shows a sample of source (Heart) and target (Intestine) tissues in MIRD model.
By calculating accumulated activity from eq.5 for mice and then converting it to human accumulate activity from eqn. (6), the absorbed dose taken from different organs can be calculated by eq.8. By considering different organs as source and target, calculated dose for each organ is obtained by sum of absorbed doses from radiation emitted from other organs in addition to absorbed dose of itself.
In this study, specific absorption fraction for 10 source and target organs (lungs, Liver, spleen, heart, stomach, pancreas, adrenal, large and small intestines and kidneys) is calculated within an adult male MIRD phantom which is written on 1996 by researchers of Hang Yang University of South Korea, is used. In this problem, MIRD phantom of a grown male consisting of soft tissue materials, bone tissue and lungs tissue is used. The tissues with absorbed activity are applied as the radioactive volume sources. Source energies of 0.14MeV for 99mTc and 364 keV (gamma), 192 keV (beta) for 131I and F6 and *F8 tallies were used for calculating internal absorbed dose. After injecting each of the radiopharmaceuticals, the mice were sacrificed and their organs dissected. A gamma counter was used to determine the percentage of injected dose per gram of organs at different times.
In continuation, by converting the mice results to the human and having accumulated activity using two methods, absorbed dose of different organs of body were obtained. On first stage by using MIRD and S factor, equivalent absorbed dose and effective absorbed dose of different organs after injection MAG3 and IOH were obtained which is shown on Tables 1-3.
| The type of radiation | Radiation weighting factor |
|---|---|
| Alpha particles | 20 |
| Beta particles | 1 |
| Gamma & X Ray | 1 |
Table 1. Weighting factor of some radiations.
| Target organs | Estimated absorbed dose (mGy/MBq) |
WT | Effective absorbed dose (mSv/MBq)a | Human Absorbed Dose mGy/MBq (Stabin et al.[5]) |
|---|---|---|---|---|
| Liver | 1.40E-04 | 0.04 | 5.58E-06 | 3.50E-04 |
| Large Intestine | 2.94E-04 | 0.12 | 3.53E-06 | 2.10E-03 |
| Pancreas | 2.03E-04 | 0.01 | 2.03E-06 | 4.50E-04 |
| Kidneys | 1.04E-03 | 0.01 | 1.03E-05 | 4.10E-03 |
| Spleen | 7.49E-04 | 0.01 | 7.49E-06 | 4.10E-04 |
| Heart | 3.23E-04 | 0.01 | 3.23E-06 | 1.80E-04 |
| Lungs | 2.23E-04 | 0.12 | 2.68E-05 | 1.50E-04 |
| Stomach | 2.81E-04 | 0.12 | 3.38E-05 | 4.50E-04 |
| Adrenals | 1.96E-04 | 0.01 | 1.96E-06 | 4.40E-04 |
aIf injected 1MBq activity of radio-tracer.
Table 2. Assessment of human effective dose based on mouse data after intravenous administration of 99mTc- MAG3 using MIRD method and comparison with stabins’s human data.
| Target organs | Estimated absorbed dose | WT | Effective absorbeda | Human Absorbed Dose mGy/MBq (Stabin et al.[5]) |
|---|---|---|---|---|
| Liver | 8.12E-04 | 0.04 | 3.25E-05 | 1.70E-03 |
| Large Intestine | 1.04E-03 | 0.12 | 1.24E-04 | 8.00E-03 |
| Pancreas | 1.02E-03 | 0.01 | 1.02E-05 | 2.10E-03 |
| Kidneys | 7.80E-03 | 0.01 | 7.80E-05 | 4.50E-02 |
| Spleen | 2.52E-02 | 0.01 | 2.52E-04 | 2.00E-03 |
| Heart | 2.50E-02 | 0.01 | 2.50E-04 | 9.00E-04 |
| Lungs | 1.36E-02 | 0.12 | 1.63E-03 | 7.70E-04 |
| Stomach | 2.41E-02 | 0.12 | 2.89E-03 | 2.10E-03 |
| Adrenals | 7.83E-04 | 0.01 | 7.83E-06 | 2.10E-03 |
aIf injected 1MBq activity of radio-tracer.
Table 3. assessment of human effective dose based on mouse data after i.v administration of 131I-Hippuran using MIRD method and comparison with stabins’s human data.
MCNP as a Monte Carlo code needs the source for a problem to be specified in a user defined input file. In the source card, particle type, source position and distribution, energy, and direction of starting particles were specified. For simulation of our work, it is assumed that the radiopharmaceuticals were uniformly distributed throughout the organs. The radiopharmaceuticals used in this research emit gamma (99mTc) and beta-gamma (99mTc, 131I) radiation. Since, Beta particles emitted from 131I have a broad energy spectrum from zero to the maximum energy so in this simulation the mean beta energy of beta particles was applied.
Tallies of F6 and *F8 can be applied for calculation of absorbed dose in MCNP code. In this simulation, both *F8 and F6 was used to calculate absorbed dose in the mentioned tissues (F6 tally only for gamma source was used).
Then human phantom was stimulated on MCNP code and the amount of effective absorbed dose of different organs for these radiopharmaceuticals was shown in Tables 4 and 5. To reduce the statistical error (below 5%), 1E7 particles were determined for NPS. A sample of geometry of Human body, with volume source located in liver tissue (planes xy and xz) is shown in Figure 2.
| Target organs | Estimated absorbeda | WT | Effective absorbed dose |
|---|---|---|---|
| Liver | 1.06E-04 | 0.04 | 4.24E-06 |
| Large Intestine | 3.35E-04 | 0.12 | 4.02E-05 |
| Pancreas | 3.02E-04 | 0.01 | 3.02E-06 |
| Kidneys | 1.16E-03 | 0.01 | 1.16E-05 |
| Spleen | 9.33E-04 | 0.01 | 9.33E-06 |
| Heart | 2.45E-04 | 0.01 | 2.45E-06 |
| Lungs | 5.93E-04 | 0.12 | 7.12E-05 |
| Stomach | 3.70E-04 | 0.12 | 4.44E-05 |
| Adrenals | 1.76E-04 | 0.01 | 1.76E-06 |
aIf injected 1MBq activity of radio-tracer.
Table 4. Assessment of human effective dose based on mouse data after i.v administration of 99mTc- MAG3 using MCNP method.
| Target organs | Estimated absorbeda | WT | Effective absorbed dose |
|---|---|---|---|
| Liver | 7.78E-04 | 0.04 | 3.11E-05 |
| Large Intestine | 1.45E-03 | 0.12 | 1.74E-04 |
| Pancreas | 1.95E-03 | 0.01 | 1.95E-05 |
| Kidneys | 9.16E-03 | 0.01 | 9.16E-05 |
| Spleen | 2.96E-02 | 0.01 | 2.96E-04 |
| Heart | 2.71E-02 | 0.01 | 2.71E-04 |
| Lungs | 1.08E-02 | 0.12 | 1.30E-03 |
| Stomach | 5.13E-02 | 0.12 | 6.16E-03 |
| Adrenals | 1.00E-03 | 0.01 | 1.00E-05 |
aIf injected 1MBq activity of radio-tracer.
Table 5. Assessment of human effective dose based on mouse data after i.v administration of 131I-Hippuran using MCNP method.
2. Results
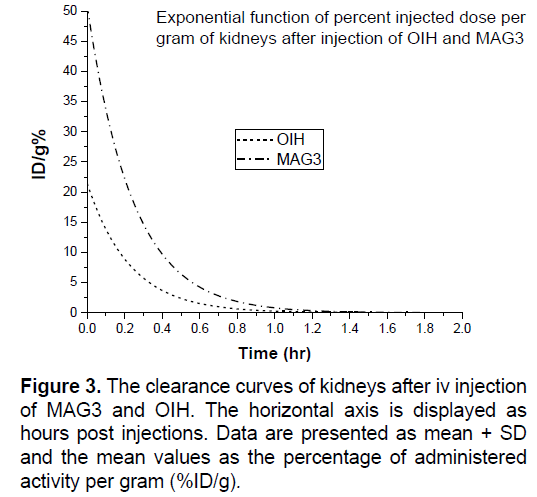
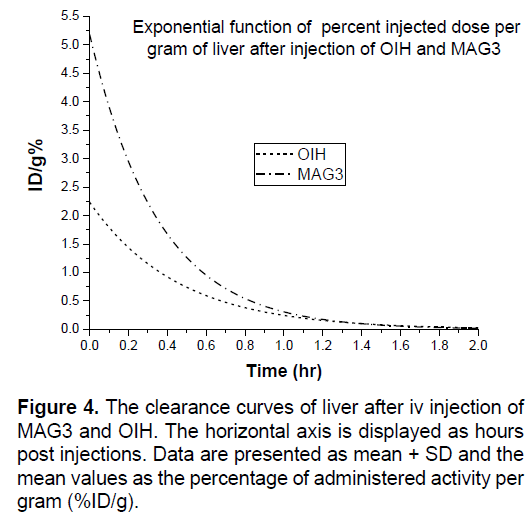
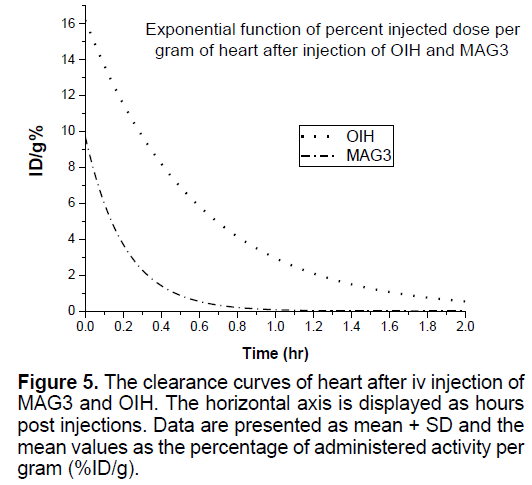
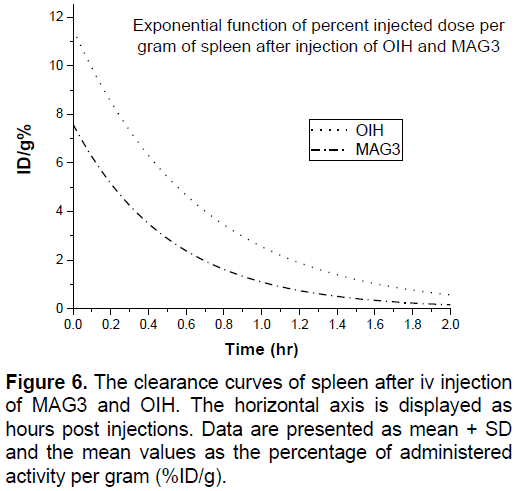
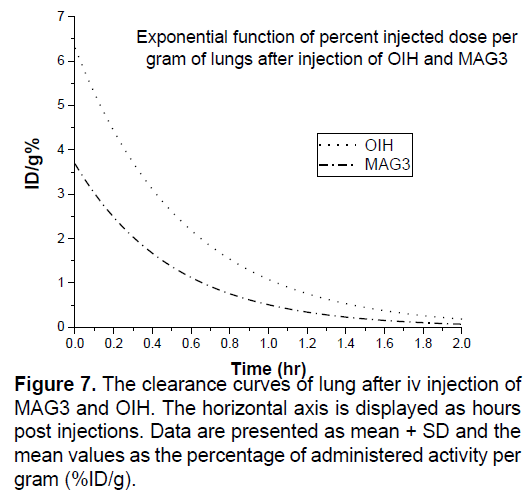
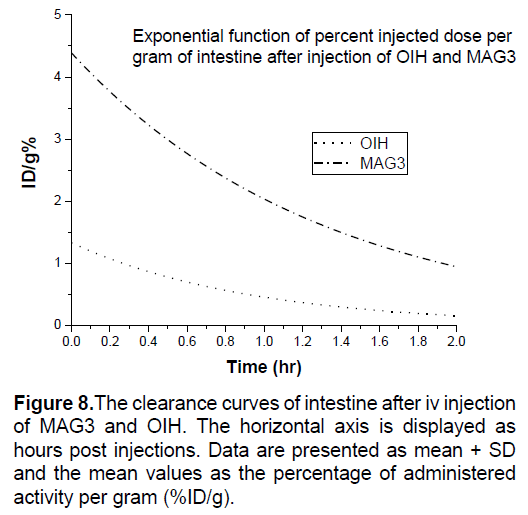
In Figures 3-8 shows the percentage of injected dose per gram at times of 10, 30 and 120 minutes after intravenous injection of 99mTc-MAG3 and OIH on different organs of mice.
After calculation of absorbed dose by MIRD and MCNP methods, the amount of effective absorbed dose of different organs for these radiopharmaceuticals was obtained. The results of both methods are compared in Tables 1-5. Tables 2 and 4 show the assessment of human effective dose from mouse data after i.v administration of 99mTc- MAG3. Tables 3 and 5 show the assessment of human effective dose from mouse data after i.v administration of 131I-Hippuran.
3. Discussion
Radiopharmaceutical renal studies provide information on renal function and structure. The information extracted from the study depends on the biological properties of the radiopharmaceuticals. Two important radiopharmaceuticals for renal system study are 99mTc- MAG3 and 131I-Hippurane. 99mTc- MAG3 was introduced by Fritzberg et al. [22,23]. The biological characteristics of the 99mTc-MAG3 are somewhat different from those of 131I-Hippurane which has considerable characteristics as effective renal plasma flow radio-agent [24]. However, 99mTc- MAG3 is now widely applied as an alternative to 131I-Hippurane for the investigation of nephrological diseases.
In this study, internal radiation dosimetry of the two mentioned renal imaging agents were calculated and compared (based on mouse data). The use of a radiopharmaceutical requires calculation of its biodistribution in animal models prior to its clinical applications [15,25,26], based on the assumption that the biodistribution could be similar in mice and in man (consistent with the recommendations of ICRP 103). Additional assessment of absorbed dose in human body from small animal such as mice may be useful for accelerating the planning of new radioactive compounds to be used in clinical experiments (recommendations of ICRP 62). There are various methods to estimate the absorbed dose after radiopharmaceuticals injection, such as MIRD, Monte carlo methods, image based dosimetry, etc.
Internal absorbed dose was calculated by using MIRD method and MCNPX simulation code, from injection of 99mTc- MAG3 and 131I-Hippuran radiopharmaceuticals on human organs resulted from accumulation of radiopharmaceutical on organs of lungs, Liver, spleen, heart, stomach, pancreas, adrenal, large and small intestines and kidneys based on distribution of radiopharmaceutical on normal mice.
The results of 99mTc-MAG3 and 131I-Hippuran distribution in body showed that the absorbed dose in spleen, lung, heart and stomach are considerable. This may be related to the chemical structure and stability of radiopharmaceuticals. The highest effective doses on MIRD and MCNP method are related to stomach and lung after injection of 1 MBq of radiopharmaceutical (131I-Hippuran). The obtained results from MIRD method were in line with MCNP results, and the difference between absorbed dose in each organ was smaller than 10 percent.
According to the results, the mouse model dosimetry (MIRD method) was not in good agreement with Stabin’s dosimetry data. It can be due to different uncertainties in both methods, small organs of mouse, different measurement instruments etc. Based on equation 6, one of the factors of the difference the obtatined results and Stabil’s results could be due to mouse to human conversion factor (Sparks and Idogan equation). Another reason for the differences between our results and Stabin’s results might be because of the difference between human and mouse tissues structure. According to the mean effective half-life of both radiopharmacuticals which is reported to be short in body, it was obvious that all the injected activity should be cleared from the main organs in short time and it would be better to have more time points before one-hour to get better results for calculationg the cumulative activity, therefore, the selection of low time points because of some limitations might be one of the reasons of difference between mouse and Stabin’s model results.
The difference between the dosimetry results (Mouse model and Stabin’s model) for 131I-Hippuran is greater than 99mTc-MAG3. The high energy gamma and beta of 131I in comparison with the gamma energy of 99mTc as well as the small organs of the mouse can be the important factors in this difference.
In general, dosimetry results of both mentioned radiopharmaceuticals show that stomach and lung received the high effective absorbed dose among under risk organs after imaging with 99mTc- MAG3 and 131I-Hippuran. Also 99mTc-MAG3 radiopharmaceutical is a better choice relative to 131I-Hippuran for renal imaging in terms of dosimetry considerations. In addition, there is no good agreement between mouse model and Stabin’s human model for 131I-Hippuran.
4. Conflict of Interest
The authors declare that they have no conflict of interest.
5. Financial Support
This research was supported by Radiation Application Research School of Nuclear Science and Technology Research Institute (NSTRI).
Assessment of Internal Absorbed Dose in the Human Abdominal Organs from Two Renal Radiopharmaceuticals Based on Experimental Mouse Data
References
- Taylor A, Clark S, Ball T. (1994). Comparison of Tc-99m MAG3 and Tc-99m DTPA scintigraphy in neonates. Clin Nucl Med. 19: 575-580.
- Shikano N, Kanai Y, Kawai K, et al. (2004). Transport of 99mTc-MAG3 via rat renal organic anion transporter 1. J Nucl Med. 45: 80-85.
- O Reilly P, Aurell M, Britton K, et al. (1996). Consensus on diuresis renography for investigating the dilated upper urinary tract. Radionuclides in Nephrourology Group. Consensus Committee on Diuresis Renography. J Nucl Med. 37: 1872-1876.
- Gordon I, Colarinha P, Fettich J, et al. (2001). Guidelines for standard and diuretic renography in children. Eur J Nucl Med. 28: BP21-BP30.
- Stabin MG, Stubbs J, Toohey R. (1996). Radiation dose estimates for radiopharmaceuticals. Rad Internal Dose Inf Center. 37831-0117.
- Erbslöh-Möller B, Dumas A, Roth D, et al. (1991). Furosemide-131I-hippuran renography after angiotensin-converting enzyme inhibition for the diagnosis of renovascular hypertension. Am J Med. 90:23-29.
- Blaufox MD, Aurell M, Bubeck B, et al. (1996). Report of the Radionuclides in Nephrourology Committee on renal clearance. J Nucl Med. 37:1883-1890.
- Eshima D, Taylor A Jr. (1992). Technetium-99m (99mTc) mercaptoacetyltriglycine: update on the new99mTc renal tubular function agent. Semin Nucl Med. 22: 61-73.
- https://www.iaea.org/inis/collection/NCLCollectionStore/_Public/22/084/22084035.pdf?r=1.
- Lahooti A, Shanehsazzadeh S, Jalilian AR, et al. (2013), Assessment of effective absorbed dose of 111In-DTPA-Buserelin in human on the basis of biodistribution rat data. Radiat Prot Dosimetry. 154: 1-8.
- Shanehsazzadeh S. (2013). Estimated background doses of [67Ga]-DTPA-USPIO in normal Balb/c mice as a potential therapeutic agent for liver and spleen cancers. Nucl Med Commun. 34: 915-925.
- Snyder W, Ford M, Warner G, et al. (1975). 'S' Absorbed dose per unit cumulated activity for selected radionuclides and organs. (MIRD pamphlet no. 11) Society of Nuclear Medicine, New York.
- Hindorf C, Glatting G,Chiesa C, et al. (2010). EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry. Eur J Nucl Med Mol Imaging. 37: 1238-1250.
- Shahbazi-Gahrouei D, Cheky M,Moslehi M. (2012). Estimation of organ absorbed doses in patients from 99mTc-diphosphonate using the data of MIRDose software. J Med Signals Sens. 2: 231-2314.
- Shanehsazzadeh S, Lahooti A, Shirmardi SP, et al. (2015). Comparison of estimated human effective dose of 67Ga-and 99mTc-labeled bombesin based on distribution data in mice. J Radioanal Nucl Chem. 305: 513-520.
- Keleher A, Wendt R, Delpassand E, et al. (2004). The Safety of Lymphatic Mapping in Pregnant Breast Cancer Patients Using Tc99m Sulfur Colloid. Breast J 10:492-495.
- https://permalink.lanl.gov/object/tr?what=info:lanl-repo/lareport/LA-13709-M
- https://www.icrp.org/publication.asp?id=ICRP%20Publication%2080
- Lazarine, Alexis D. (2006). Medical physics calculations with MCNP: a primer. Master's thesis, Texas A&M University.
- Shanehsazzadeh S, Lahooti A, Yousefnia H. et al. (2015). Comparison of estimated human dose of 68Ga-MAA with 99mTc-MAA based on rat data. Ann Nucl Med.29: 745.
- Shanehsazzadeh S, Yousefnia H, Jalilian AR. et al. (2015). Estimated human absorbed dose for 68Ga-ECC based on mice data: comparison with 67Ga-ECC. Ann Nucl Med.29: 475.
- Fritzberg AR, Kasina S, Eshima D, et al. (1986). Synthesis and biological evaluation of technetium-99m MAG3 as a hippuran replacement. J Nucl Med. 27: 111-116.
- Taylor A Jr, Eshima D, Fritzberg AR, et al.(1986). Comparison of iodine-131 OIH and technetium-99m MAG3 renal imaging in volunteers. J Nucl Med. 27: 795-803.
- Itoh K. (2001). 99mTc-MAG3: Review of pharmacokinetics, clinical application to renal diseases and quantification of renal function. Ann Nucl Med. 15: 179-190.
- Rezaeejam H, Hakimi A, Jalilian AR, et al. (2015). M Determination of human absorbed dose from [153Sm]-Samarium maltolate based on distribution data in rats. Int J Radi Res. 13:173-180.
- Pirdamooie S, Shanei A, Moslehi M. (2015). Comparison of the Absorbed Dose for 99mTc-Diethylenetriaminepentaacetic Acid and 99mTc-Ethylenedicysteine Radiopharmaceuticals using Medical Internal Radiation Dosimetry. J Med Signals Sens. 5: 171-175.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences