An Overview of Mechanism of Egress of RBC from Bone Marrow
Fatima Zafar*, Razia Iqbal, Muhammad Faheem Malik, Muhammad Irfan, Mubashar Hussain
1Department of Zoology, University of Gujrat, Pakistan
2Department of Biotechnology, University of Sargodha, Pakistan.
- *Corresponding Author:
- Fatima Zafar
Department of Zoology, University of Gujrat
Pakistan
E-mail: fatimazafarpu@gmail.com
Received: November 15, 2018; Accepted: May 13, 2019; Published: May 20, 2019
Citation: Zafar F, Iqbal R, Malik MF, et al. An Overview of Mechanism of Egress of RBC from Bone Marrow. Electronic J Biol, 15:2
Abstract
Hematopoietic stem cell in bone marrow committed to produce erythrocyte- the red blood cells. Once mature these cells continuously egress out into general circulation to perform their normal function in the body. Bone marrow generally separated from peripheral blood via sinusoid membrane that has small pores in its endothelial layer. RBCs egress out through specific sites (pores) from endothelial layer. During maturation process RBCs lose its nucleus and became discoid shape. That imparts the greater deformability into them so they could easily squeeze out through marrow aperture. The egress of RBCs further accelerated as a result of humoral factors (releasing factors) i.e. stress, cytokines, chemokines, erythropoietin and proteolytic enzymes like GTPases.
Keywords
Bone marrow; Sinusoid membrane; Deformability; Humoral factors.
1. Introduction
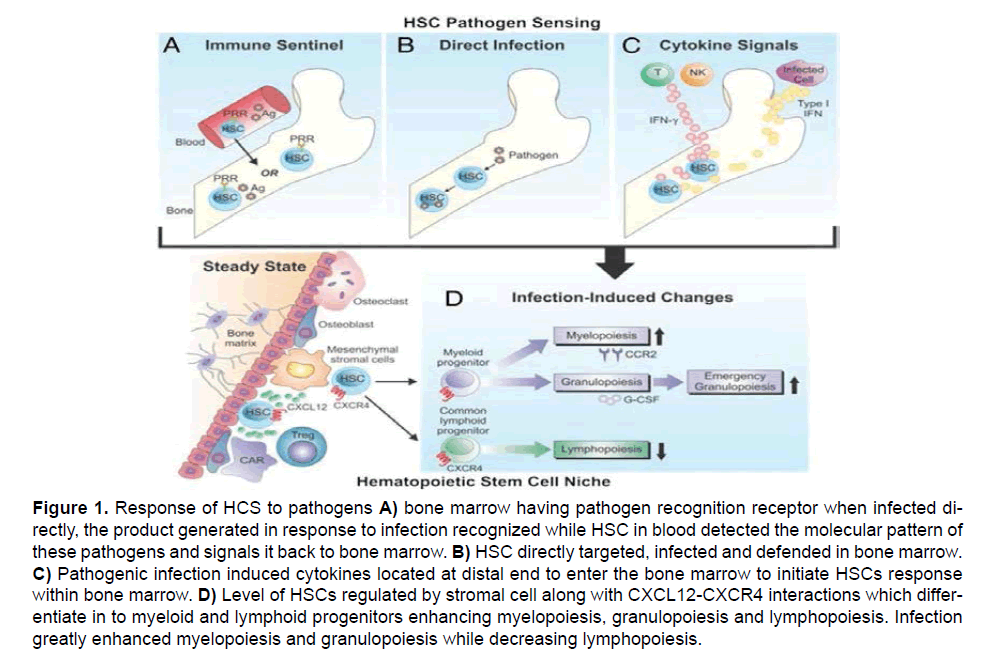
Bone marrow is considered as a pool of hematopoietic stem cells of different morphology and characteristics, which produce erythrocytes and leucocytes etc. Hematopoietic stem cells (HSCs) in bone marrow mainly resides in between the medullary bone and stromal cells [1]. The erythrocytes produced by HSCs have to come out from bone marrow to general circulation to perform their normal functions. Hematopoietic stem cells might be gets into general circulation helpful in defense mechanism [2-7]. The mechanism of egress of erythrocytes- the red blood cell from bone marrow is determined by many factors like the restraining barrier between bone marrow and sinusoid, pore size in the membrane, cell’s ability to cross these pores and influence of certain releasing factors which enhance the passage of cells to pores of smaller diameter [8-11]. Egress mainly enhanced under stress situations i.e., anxiety and exercise [12]. Depending upon concentration of RBC in general circulation, bone marrow proliferates to produce new RBCs and release them to general circulation to accommodate the requirements of the body. This is called as feedback mechanism that is completed when the old RBCs in general circulation die and warn out after completing their life span of 120 days. However, in the pathological condition like Malaria, Toxoplasmosis or in case of genetic defects the normal feedback system get disturbed by increasing granulocytes in bone marrow and decreasing erythrocytes and lymphocytes, disturbing the overall egress of RBCs [13-16] as shown in Figure 1. After parasitic infection decrease erythropoiesis lead to anemia [17] i.e., Plasmodium, Trypanosoma and Babesia sp., directly lead to the destruction of erythrocyte precursor and ultimately leading to reduction in total RBCs count [18].
Figure 1: Response of HCS to pathogens A) bone marrow having pathogen recognition receptor when infected directly, the product generated in response to infection recognized while HSC in blood detected the molecular pattern of these pathogens and signals it back to bone marrow. B) HSC directly targeted, infected and defended in bone marrow. C) Pathogenic infection induced cytokines located at distal end to enter the bone marrow to initiate HSCs response within bone marrow. D) Level of HSCs regulated by stromal cell along with CXCL12-CXCR4 interactions which differentiate in to myeloid and lymphoid progenitors enhancing myelopoiesis, granulopoiesis and lymphopoiesis. Infection greatly enhanced myelopoiesis and granulopoiesis while decreasing lymphopoiesis.
1.1.Egress of erythrocytes through sinusoid membrane
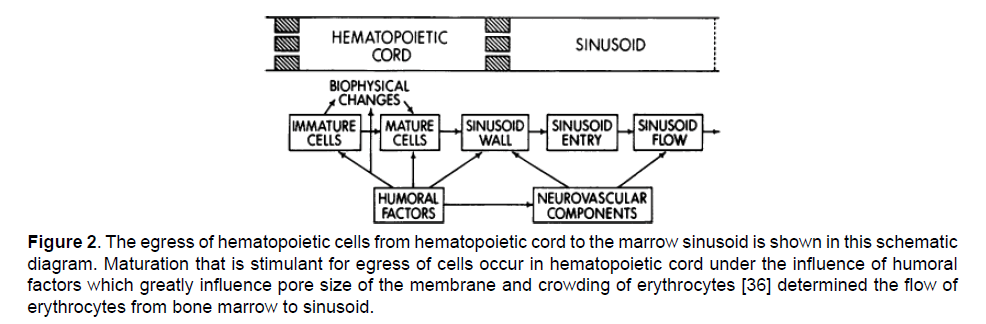
Erythrocytes egress from hematopoietic cell to marrow sinusoid. Structure of marrow sinusoid under electron microscope reveals a trilaminar structure i.e., the adventitial cell towards marrow cord, a middle basement membrane and endothelial lining toward sinusoid containing pores [19]. Adventitial cell having the microfilaments structure provide area for deformed mature cells. Only the pores of endothelial allow the egress of RBCs. The pores of endothelial cell in the marrow sinusoid has diameter smaller than that of the cells [20-23]. Depending upon external requirement of erythrocytes, the marrow cell proliferate rapidly to produce greater number of erythrocytes which mature quickly and come out to general circulation to retain the steady state of body [24-27]. The egress of marrow cell when observed by placing the cells in a small millipore filters with diameter of 1 to 8 um revealed that the rate of marrow cell egress greatly increased by increasing the pore size and by the application of chemoattractants - releasing factors. Studies indicate that only mature erythrocytes have the ability to cross the pores of barrier membrane as compared to immature cells [28] due to their deformability properties and de-nucleation [29-31]. However, some immature cells also come out into general circulation along with these mature cells [14]. The mature cells also respond rapidly to chemical attractants to come out into general circulation [8,32] (Figure 2).
Figure 2: The egress of hematopoietic cells from hematopoietic cord to the marrow sinusoid is shown in this schematic diagram. Maturation that is stimulant for egress of cells occur in hematopoietic cord under the influence of humoral factors which greatly influence pore size of the membrane and crowding of erythrocytes [36] determined the flow of erythrocytes from bone marrow to sinusoid.
1.2. RBCs egress through specific sites in marrow sinuses
The egress of reticulocytes considered first by thinning and formation of depression in the sinusoid wall after the maturation of reticulocytes [33-37]. Erythropoiesis occurs in bone marrow and at those sites’ sinusoid wall penetrate and receive RBCs, moving them to central sinuses followed by marrow vein to general circulation [38]. RBCs penetrate at specific thin parajunctional sites (pores) in sinusoid wall where they encounter no resistance for crossing. This initial interaction between endothelial cells and basement membrane resulted in large number of aperture formation. These pores open only at the time of egress of RBCs while close otherwise suggesting egress of RBCs occur only at specific sites in specific time through marrow sinusoid [37].
1.3. Deformability enhance egress of RBCs
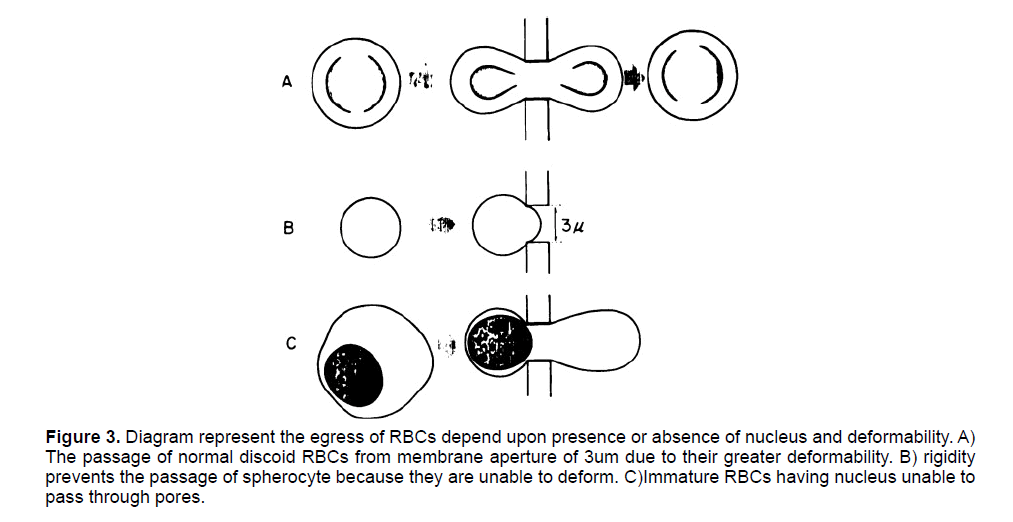
The egress of RBC is mainly determined by their shape (Discoid), presence or absence of nucleus, and properties of the membrane through which they have to pass [39]. The discoid shape and the absence of nucleus offer rapid deformability in the mature erythrocytes for their egress from bone marrow. While the immature cells cannot deform due to their shape and presence of nucleus which impart rigidity in these cells hence, they are unable to cross the sinusoid pores [28]. On the other hand, a high deposition of Ca2+ and Mg2+ in membrane makes the membrane rigid so the egress is no longer possible [40] (Figure 3).
Figure 3: Diagram represent the egress of RBCs depend upon presence or absence of nucleus and deformability. A) The passage of normal discoid RBCs from membrane aperture of 3um due to their greater deformability. B) rigidity prevents the passage of spherocyte because they are unable to deform. C)Immature RBCs having nucleus unable to pass through pores.
The detailed structure of marrow sinusoid reveals pore sizes of 3um, in membrane separating hematopoietic chord and marrow sinusoid [41- 43]. Mature reticulocytes due to de-nucleation and discoid shape have the well enough deformability to allow them cross this microvasculature [44]. Studies suggested that the morphology of mature reticulocytes offer them greater deformability to pass through the micropipette use in laboratory which has the pores similar to that of marrow sinusoid [45,46]. However, the pathological conditions like hemolysis and shift reticulocytes resulted in rigid premature stages of erythrocytes to egress out into general circulation [28].
2. Stimulants and egress of RBCs
In certain pathological conditions when certain stimuli are injected like Vit B12 (anemia), Haematinics (haemorrhage), cobalt (Defects in pulmonary diffusion) and iron (iron deficiency anemia), the rate of production of reticulocytes increase exponentially irrespective of the concentration of RBCs in general circulation [47]. While the administration of Sodium carbonate and adrenaline (air encephalography) resulted in increased release of erythrocytes
Administration of erythropoietin, adrenocorticotropic hormone cytotoxic drugs along with stress, bleeding and intense physical exercise significantly increase egress of RBCs [48-51].
2.1. Effect of stress on RBCs egress
Stress stimulate the release of certain chemicals, cytokines and the proteolytic enzymes that modulate intrinsic mechanisms inducing the motility and egress of RBCs from bone marrow’s specific sites [52,53] demonstrate that elevated stress conditions i.e. acute inflammation resulted in increased activity of the Hematopoietic cells to proliferate and produce greater number of RBCs permitting them to pass through sinusoid aperture [4].
2.2. Effect of internal signaling-GTPases on egress of RBCs
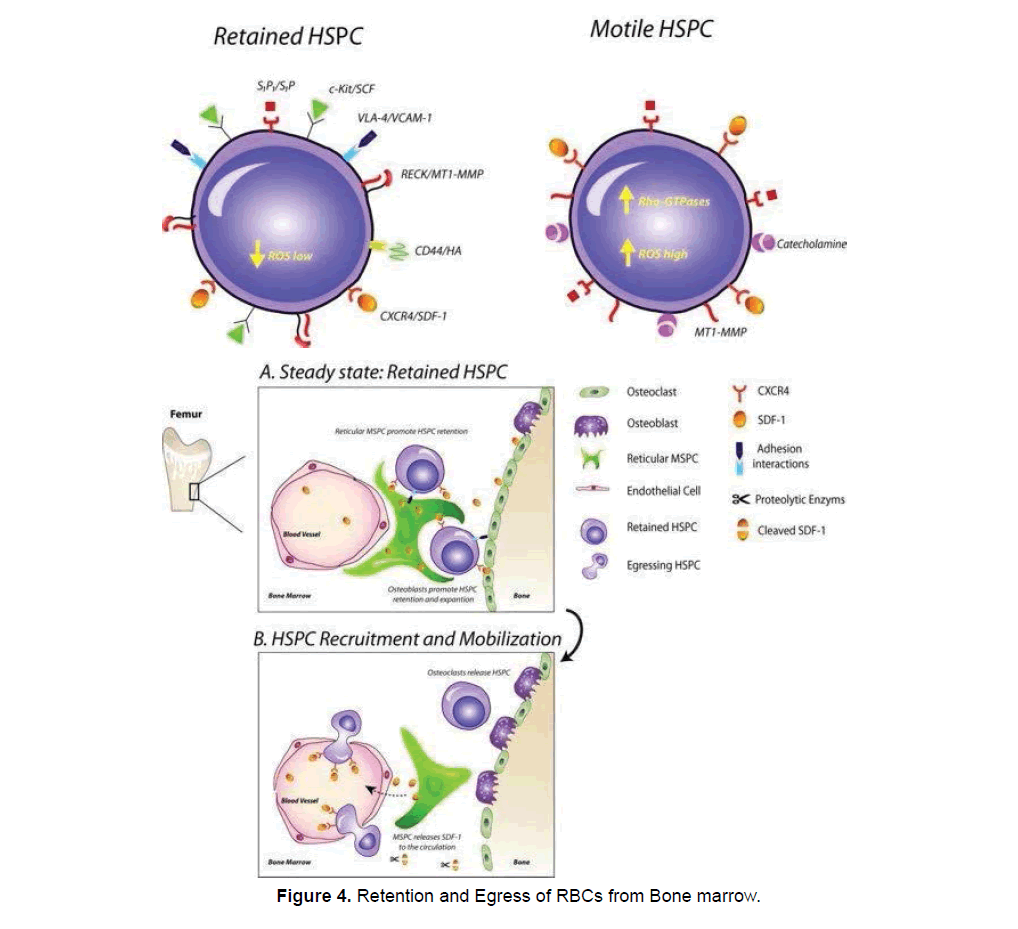
Guanosine triphosphatases is an internal signaling enzyme that modulates the active process of hematopoietic cell proliferation and egress [54]. Different chemokines and cytokines i.e. SDF-1/ CXCR4 gets activated by the GTPase signaling [55]. CXCL12 a cytokine important for maintaining HSCs population in bone marrow, disturbance in interaction between CXCL12-CXCR4 leading to premature HSCs release from bone marrow [56,57]. Cdc42, Rho A, Rac1 and Rac2 respond strongly towards GTPase based chemokines and cytokines signals to accelerate the process of RBCs egress [58-61]. Stomatal cell derived Factor (SDF) a cytokine along with mobilization of RBC has important roles in retention, and survival. Various bone marrow stromal niche cells i.e., endosteal bone lining osteoblasts [62] and bone marrow endothelium [63] express the SDF. Recently hypothesized that administration of AMD3100 interrupts SDF stimulation that leaded to RBC retention.
2.3.Effect of cytokines and chemokines on RBCs egress
Granulocyte colony stimulating factor (G-CSF)–a cytokine and most widely used hematopoietic egress inducing agent [64]. And the excessive repeated administration of G-CSF enhances mobilization of bone marrow contents into general circulation [65]. G-CSF gets activated by adrenergic stimulation [66]. Administration of AMD3100-a chemokines either singly or in combination with G-CSF enhance the mobilization of HSCs [67-70] reported that the AMD300 cause diminishing SDF-1/CXCR4 (fucoidan and catecholamine) interaction in bone marrow causing their elevated concentration of SDF-1 in peripheral blood. This loosen interaction between SDF-1/CXCR4 facilitates increase erythrocyte egress from bone marrow [71-73]. Similarly, another chemokine T-140 enhance egress of hematopoietic cells from bone marrow [74,75] reported that in contrast to G-CSF cytokine which requires daily administration the cytokines like CXCR2 ligand GROβ by secreting certain wall digesting proteolytic enzymes enable egress within 20 minutes. The whole process of RBCs egress under the influence of different external and internal stimulants shown in Figure 4.
Under normal condition hematopoietic cells product (reticulocytes) retained in bone marrow in retention condition and only a small fraction of cell is freely circulating in general circulation. SDF-1 in bone marrow permit them to retain there while under stress condition level of G-CSF along with AMD3100 increased which greatly accelerate the process of egress. Different huomoral factors act on the membrane surface to greatly enhance the egress mechanism [52].
2.4. Erythropoietin, bone remodeling and egress of RBCs
Endogenous and exogenous erythropoietin administration resulted in increased egress of RBCs by increasing the number of pores in sinusoid membrane, decreasing the cells of adventitial layer and diminution of hematocrit cells. But the excessive administration of erythropoietin counter acts this effect due to increase hematocrit level [76,77]. Osteoblasts (by MSPC) and osteoclasts (by monocyte) established bone equilibrium within endosteum in the vicinity of HSCs [78]. Bone formation and degeneration disturb the stem cells niches and ultimately affect the HSCs balance in bone marrow. Administration of Granulocyte colony stimulating factor (G-CSF) or cyclophosphamide injections altered the morphology of osteoblasts by expanding it and promoting HSCs proliferation, resulting in reduced transcription of SDF-1, and VCAM-1 thus HSCs loss their retention [79-83].
3. Conclusion
Overall, it is concluded from the review of literature that the maturation process enhances the deformability of normal discoid shape erythrocytes. This deformability permits them to easily pass through the pores in sinusoid membrane. The hematopoietic cells respond strongly to the certain humoral factor that increases their permeability through the membrane by increasing pore size. But the onset of infection from pathogen and parasites disturb the dynamics of HSCs and ultimately reduce the RBC egress. Certain chemokines and cytokines i.e., G-CSF, AMD3100, CXCR12 and SDF deliberately enhance RBC egress from bone marrow. CXCR2 ligand GROβ by secreting certain wall digesting proteolytic enzymes enable egress within 20 minutes.
4. Acknowledgement
We would like to acknowledge the technical and moral support of Mr Muhammad Awais Zafar during writing and compiling this review.
References
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 481:457-462.
- Yang M, Büsche G, Ganser A, Li Z. (2013). Morphology and quantitative composition of hematopoietic cells in murine bone marrow and spleen of healthy subjects. Ann Hematol. 92:587-594.
- Travlos GS. (2006). Normal structure function and histology of the bone marrow. Toxicol Pathol. 34:548-565.
- Zaretsky AG, Engiles JB, Hunter CA. (2014). Infection-Induced Changes in Hematopoiesis. J Immunol. 192:27-33.
- Orkin SH, Zon LI. (2008). Hematopoiesis: an evolving paradigm for stem cell biology. Cell.132:631-644.
- Massberg S. (2007). Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 131:994-1008.
- Abkowitz JL. (2003). Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 102:1249-1253.
- Giodano FG, Lichtman AM, Mayle E. (1973). Marrow Cell Egress; The central interaction of barrier pore size and cell maturation. J Clin Invest.52: 1154-1164.
- Marsh JC, LeVitt M. (1971). Neutrophilic-inducing activity in plasma of neutropoenic human beings. Blood J Heinatol. 37: 647.
- Boggs DRGE, Cartwright MM, Wintrobe. (1966). Neutrophilia inducing activity in plasma of dogs recovering from drug induced myelotoxicity. Ami J Phv Siol. 211: 51.
- Gordon ASJ, LoBue BS, Dornfest GM, Cooper. (1962). Retriculocyte and leukocyte release from isolated perfused rat legs and femurs. Erythropoiesis. New York. 321.
- Kroepfl JM. (2012). Exercise Increases the Frequency of Circulating Hematopoietic Progenitor Cells, But Reduces Hematopoietic Colony-Forming Capacity. Stem Cells Dev. 21:2915-2925.
- Savill NJ, Chadwick W, Reece SE. (2009). Quantitative Analysis of Mechanisms That Govern Red Blood Cell Age Structure and Dynamics during Anaemia. PLoS Comput Biol 5: e1000416.
- Lapidot T, Petit I. (2002). Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 30:973-981.
- Villeval JL, Gearing A, Metcalf D. (1990). Changes in hemopoietic and regulator levels in mice during fatal or nonfatal malarial infections. II. Nonerythroid populations. Exp Parasitol. 71:375-385.
- Villeval JL, Lew A, Metcalf D. (1990). Changes in hemopoietic and regulator levels in mice during fatal or nonfatal malarial infections. I. Erythropoietic populations. Exp Parasitol. 71:364-374.
- Stein BL. (2012). The anemia of inflammation. J Clin Rheumatol. 18:437-442.
- [18]Nishimura K, Nakaya H, Nakagawa H, et al. (2011). Effect of Trypanosoma bruceibrucei on erythropoiesis in infected rats. J Parasitol. 97: 88-93.
- Weiss L. (1965). The structure of bone marrow: functional interrelationships of vascular and hemopoietic compartments in experimental hemolytic anemia: an electron microscopic study. J. Morph. 117:467.
- DeBrulyin PPHS, Michelson TB, Thomas. (1971). The migration of blood cells of the bone marrow through the sinusoidal wall. J Morphol. 133:417.
- Trubowitz S, Masen B. (1970). The structural organization of the human marrow matrix in thin sections. Anm J Clin Pathol. 53:908.
- Weiss L. (1970). Transmural cellular passage in vascular sinuses of rat bone marrow. Blood J Heniatol. 36:189.
- Weiss L. (1961). An electron microscopic study of the vascular sinuses of the bone marrow of the rabbit. Bull Johnis Hopkins Hosp. 108:171.
- King Smith EA, Morley A. (1970). Computer stimulation of granulopoiesis: normal and impaired granulopoiesis. Blood J Hematol. 36:254.
- Cronkite EP, Vincent PC. (1970). Granulocytopoiesis. In Hematopoietic Cellular Proliferation. Stohlman F, Jr., editor. Grune & Stratton, Inc., New York. 2:11-17.
- Stohlman F Jr. (1967). Some aspects of erythrokinetics. Semin. Hematology. 4:304.
- Fisher JWLG, Lajtha AS, Buttoo DD, Porteous. (1965). Direct effects of erythropoietin on the bone marrow of the isolated perfused hind limb of rabbits. Br J Haematol. 11:342.
- LeBlond PPL, LaCelle Weed RI. (1971). Cellular deformability: a possible determinant of the normal release of maturing erythrocytes from bone marrow. Blood J Hernatol. 37:40.
- Lichtman MA, Chamberlain JK, Weed RI, et al. (1977). The regulation of the release of granulocytes from normal marrow, inGreenwalt TJ, Jamieson GA (editors): The Granulocyte: Function and Clinical Utilization. New York, Liss, 53.
- Katz RAS, Gordon DM, Lapin. (1966). Mechanisms of leukocyte production and release. VI. Studies on purification of the leukocytosis-inducing factor. J Reticulocndothcl Soc 3:103.
- Lapin DMJ, LeBue AS, Gordon ED Zanjani, et al. (1969). Mechanisms of leucocyte production and release IX. Kinetics of leucocyte release in leu cocytophoresed rats. Proc Soc Exp Biol Med. 131:756.
- Kass L, DeBruyn PPH. (1967). Chemotaxis of mature and immature blood cells in tissue cultures. Anat Rec. 159:115.
- Lichtman MA, Weed RI. (1972). Alteration of the cell periphery during granulocyte maturation: relationship to cell function. Blood J Hemnatol. 39:301.
- Lichtman MA. (1970). Cellular deformability during maturation of the myeloblast. Possible role in marrow egress. N Eiygl J Med. 283:943.
- Rytomaa T, Kiviniemi K. (1970). Regression of generalized leukaemia in rat induced by the granulocytic chalone. Eur J Cancer. 6:401.
- Chen Li-Tsun EE, Handler ES, Handle L, et al. (1972). An electron microscopic study of the bone marrow of the rat in an experimental myelogenous leukemia. Blood J Hematol. 39:99.
- Chamberlain KJ, Lichtman AM. (1978). Marrow Cell Egress: Specificity of the Site of Penetration in to the Sinus. Blood. 52:959-968.
- DeBruyn PPH, Breen PC, Thomas TB. (1970). The microcirculation of the bone marrow. Anat Rec. 168:55.
- Rand RP, Burton AC. (1964). Mechanical properties of the red cell membrane. I. Membrane stiffness and intracellular pressure. Biophys J. 4:115.
- Weed RI, LaCelle PL, Merrill WS. (1969). Metabolic dependence of red cell deformability. J Clin Invest. 48:795.
- Wantanabe Y. (1966). An electron microscopic study of the reticuloendothelial system in the bone marrow. Tohoku J ExpMed. 89:167.
- Zamboni L, Pease DC. (1961). The vascular bed of red bone marrow. J Ultrastruct Res.
- Bessis M, Breton-Corius J. (1960). Diapesis of reticulocy et des erythroblastes. (Diapedesis of reticulocytes and erythroblasts.) C. R. Acad. Sci. (Paris). 251:465.
- Weed RI. (1968). The cell membrane in hemolvtic disorders. In Jaff {233},E. (Ed.): Twelfth Congress of the International Society of Hematology, Plenary Session Papers. New York, Grune and Stratton.
- LaCelle PL. (1970). Alteration of membrane deformability in hemolytic anemias. Seminars Hemat. 7:355.
- Lichtman MA, Weed RI. (1969). Surface characteristics of human immature and mature granulocytes: relationships to granulocyte function. Blood. 34:825.
- Smith H. (1962). Reticulocyte release factor. J clin Path. 15:260.
- Zaldivar F. (2007). The effect of brief exercise on circulating CD34+ stem cells in early and late pubertal boys. Pediatr Res. 61:491-495.
- Elsenbruch S. (2006). Public speaking stress-induced neuroendocrine responses and circulating immune cell redistribution in irritable bowel syndrome. Am J Gastroenterol. 101:2300-2307.
- Kollet O (2006). Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 12:657-664.
- Lapid K, Glait-Santar C, Gur-Cohen S, et al. (2012). Egress and Mobilization of Hematopoietic Stem and Progenitor Cells: A Dynamic Multi-facet Process. Harvard Stem Cell Institute.
- Grisaru D. (2001). ARP, a peptide derived from the stress-associated acetylcholinesterase variant, has hematopoietic growth promoting activities. Mol Med. 7:93-105.
- Cancelas JA, Williams DA. (2009). Rho GTPases in hematopoietic stem cell functions. Curr Opin Hematol. 16:249-254.
- Mulloy JC. (2010). Rho GTPases in hematopoiesis and hemopathies. Blood.115: 936-947.
- Motabi IH, DiPersio JF. (2012). Advances in stem cell mobilization. Blood Rev. 26:267-278.
- Ma Q, Jones D, Springer TA. (1999). The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 10:463-471.
- Yang L, Zheng Y. (2007). Cdc42, a signal coordinator in hematopoietic stem cell maintenance. Cell Cycle. 6:1445-1450.
- Ghiaur G. (2006). Inhibition of RhoA GTPase activity enhances hematopoietic stem and progenitor cell proliferation and engraftment. Blood. 108:2087-2094.
- Jansen M. (2005). Rac2-deficient hematopoietic stem cells show defective interaction with the hematopoietic microenvironment and long-term engraftment failure. Stem Cells. 23:335-346.
- Gu Y. (2003). Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 302:445-449.
- Ponomaryov T. (2000). Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest.106:1331-1339.
- Ceradini DJ. (2004). Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 10:858-864.
- Korbling M, Anderlini P. (2001). Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 98:2900-2908.
- Bonig H. (2007). Hematopoietic progenitor cells (HPC) from mobilized peripheral blood display enhanced migration and marrow homing compared to steady-state bone marrow HPC. Exp Hematol. 35:326-334.
- Katayama Y. (2006). Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 124:407-421.
- Ferraro F. (2011). Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 3:101-104.
- Devine SM. (2008). Rapid mobilization of functional donor hematopoietic cells withoutG-CSF using AMD3100, an antagonist of the CXCR4SDF-1 interaction. Blood. 112:990-998.
- Broxmeyer HE. (2005). Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 201:1307-1318.
- Liles WC. (2003). Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 102:2728-2730.
- Dar A. (2011). Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 25:1286-1296.
- Hidalgo A. (2004). The integrin alphaMbeta2 anchors hematopoietic progenitors in the bone marrow during enforced mobilization. Blood.104:993-1001.
- Schajnovitz A. (2011). CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat Immunol. 12:391-398.
- Abraham M. (2007). Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. Stem Cells. 25:2158-2166.
- Fukuda S. (2007). The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood. 110:860-869.
- Chamberlain KJ, Weiss L, Weed IR, (1975). Bone Marrow Sinus Cell Packing: A Determinant of Cell Release. Blood. 46:91-102.
- Campbell FC. (1972). Ultrastructural studies of transmural migration of blood cells in the bone marrow of rats, mice, and guinea pigs. Am J Anat. 135:521.
- Kollet O, Dar A, Lapidot T. (2007). The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol. 25:51-69.
- Semerad CL. (2005). G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 106: 3020-3027.
- Christopher MJ. (2009) Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 114:1331-1339.
- Katayama Y. (2006). Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 124: 407-421.
- Winkler IG. (2012). Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 26:1594-1601.
- Li S. (2012). A pivotal role of bone remodeling in granulocyte colony stimulating factor induced hematopoietic stem/progenitor cells mobilization. J Cell Physiol. (Epub ahead of print).
- Mayack SR, Wagers AJ. (2008). Osteolineage niche cells initiate hematopoietic stem cell mobilization. Blood. 112: 519-531.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences