Aluminum Inhibition of ÃÆà ½Ãâñ-chymotrypsin, Acetyl Cholinesterase and Superoxide Dismutase shows a Link with Alzheimer Disease
Miriam Barquero Quiros
University of Costa Rica, CELEQ, San Pedro de Montes de Oca, 11500-2060 San José, Costa Rica.
- Corresponding Author:
- Miriam Barquero Quiros
University of Costa Rica
CELEQ, San Pedro de Montes de Oca
11500-2060 San José, Costa Rica
Tel: 506-2511-2487
Fax: 506-2511-2487
E-mail: miriam.barquero@ucr.ac.cr
Received date: November 26, 2016; Accepted date: January 06, 2017; Published date: January 13, 2017
Citation: Quiros MB. Aluminum Inhibition of α-Chymotrypsin, Acetylcholinesterase and Superoxide Dismutase shows a Link with Alzheimer Disease. Electronic J Biol, S:2
Abstract
Aluminum is present in all biospheres; it does not have any essential known function, although it had shown toxicity at high concentration towards laboratory animals, plants and humans. Its content is higher in brain patients with Alzheimer Disease (AD) and in people with renal failure. It is not clear if aluminum accumulation is a cause or consequence of AD. Due to aluminum had shown inhibition on enzymes present on human organism and these ones are related with symptoms and AD characteristic structures as neuritic plaques and neurofibrillary tangles, some studies were conducted with α-chymotrypsin, acetyl cholinesterase and superoxide dismutase enzymes. Michaelis Menten apparent constants (Kmapp) were calculated for the first-time trough Lineweaver Burke plots using screen carbon electrodes with immobilized α-chymotrypsin, acetyl cholinesterase and superoxide dismutase. Aluminum showed enzymatic inhibition at μmolar level. Competitive Kmapp increased with aluminum concentration confirming aluminum inhibition on tested enzymes. Main literature reviewed about selected enzymes, coupled with experimental evidence let us to conclude that, it is possible to set a link between these enzymes and Alzheimer disease. Three hypotheses established to explain toxic effect of metals are related with aluminum action on selected enzymes.
Keywords
Aluminum toxicity; Enzymes inhibition; α-chymotrypsin; Acetylcholinesterase; Superoxide dismutase; Alzheimer disease.
Introduction
Aluminum does not have any essential known role in living organisms. Toxicity have been reported on aquatic organisms increasing with pH lower values [1,2]. While in plants it has accumulative effect crossing membrane of terrestrial plants roots by ion bonding with a carriers such as citrate and malate [3,4]. Its presence reduces root growth and uptake of Ca and Mg, increasing rigidity of cell walls [5,6].
Exposure to aluminum leads to changes in plants genes expression, interfering with assimilation/ speciation of essential metals at cellular level, disrupting cell redox balance and increasing the accumulation of reactive oxygen species (ROS) [7]. Laboratory animals fed with Al high diets, decrease food intake, weight, Mg absorption, also Fe and ferritin burden change. These results suggest altered regulation of Fe metabolism [8,9].
Humans are exposed to aluminum contamination from natural and anthropomorphic sources, water and food are part of daily intake [10,11]. Vulnerable populations such as infants and people who use antacids or have kidney failure, are more exposed to its toxic effects [12,13]. Its accumulation increases with age and in brain’s patients with AD, high levels of aluminum were found [11,14,15]. Aluminum from various sources is complexed by food constituents in gastrointestinal tract. In this place it is absorbed in stomach or proximal duodenum, by endogenous ligands action [16,17]. Pyruvate also enhanced transport process [18]. Exogenous ligands enhance the intestinal absorption of aluminum in rats due to increased mucosal permeability [19-21]. Transported aluminum accumulates in neurons [22]. Owing to aluminum content in AD brains is higher; and ferritin is altered, it is considered interfering with normal cellular iron homeostasis disturbing associated processes in nervous system and brain [23].
Aluminum Enzyme Inhibition and Alzheimer's Disease
The first reported case of AD occurred in Frankfort where aluminum was used to refine drinking water process and features of disease were described [24]. Evidence suggests that AD patients have a higher blood aluminum level after ingestion of aluminum citrate. After 26Al administration in a glass of water, it is found in brain, supporting evidence that living brain tissue intakes aluminum from bloodstream. Therefore, brain aluminum is not an imprint of its passive absorption in diseased cells or in apoptosis process [24,25]. Its effects on DNA, protein synthesis and cholinergic neurotransmission, are similar to AD diminished functions, enhancing oxidative damage induced by Fe on hippocampal neurons, cerebellum and myelin, contributing to neurodegenerative disorders [26-28]. AD is characterized by neurofibrillary tangles and amyloid plaques, these structures obviously reduce function of acetylcholine receptors with decreased functions of dendrite, axon and synapses [29]. Aluminum inhibits scavenger enzymes of free radicals as superoxide dismutase (SOD) and glutathione peroxidase promoting oxidative cell stress.
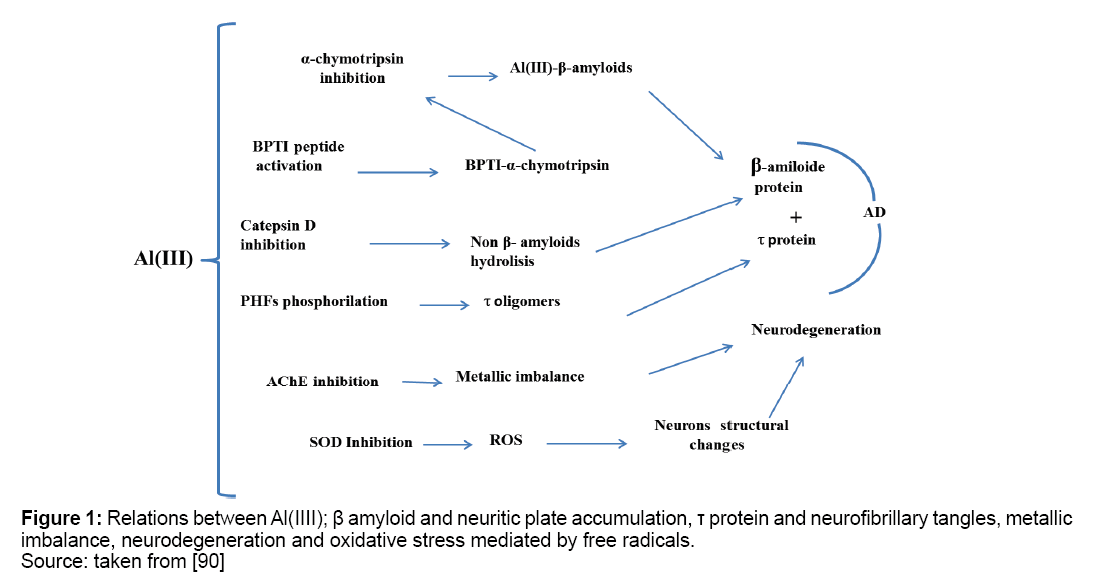
Three hypotheses related with AD have been issued for metals toxic effect as aluminum on the human body. These are amyloid cascade, metal ion and oxidative stress. It is possible to set a link between AD and some aluminum inhibited enzymes. Aluminum action on the enzyme chymotrypsin and serine group proteases resembles AD symptoms [30,31]. Aluminum enables hyper phosphorylation of proteins components from β-amyloid plaque, contributing to its aggregation [32]. In addition, to non-enzymatically manner, phosphorylates human τ protein in vitro and induces protein aggregation of pairs helical filaments (PHFs) increasing their resistance to proteolytic degradation [33]. τ normal protein is replaced by τ abnormal protein, which increases with aluminum concentration [34,35]. On laboratory animals, aluminum administration produces deficits in learning and memory related to poor metabolism of acetylcholine [36,37]. Effects of aluminum on central nervous system are inhibition of synaptic transmission and impaired synapse formation [38]. Proposed aluminum action on tested enzymes is depicted in Figure 1.
Aluminum and Amyloid Cascade Hypothesis
Amyloids proteins are the main extracellular neurotoxic substances of AD. These ones are accompanied by other intra neuronal structures named neurofibrillary tangles (NFTs) [39]. Models have been proposed to explain toxicity of Al (III), they considered aluminum inhibition of the proteolytic activity of serine proteases as trypsin and α-chymotrypsin promoting buildup of plaque β-amyloid and NFTs formation [40,41]. It has been found that injection of paired filaments of β-amyloid protein and aluminum within the brain of mice induces stable codeposits of β-amyloid and α-chymotrypsin [42]. A thermodynamic model showed that Al(III) interacts with liposomes into two binding sites, the first with the polar head of the phospholipids, and the second with peripheral sites, modifying structure membrane, inducing apoptosis in human lymphocytes [43-45]. Accumulation of protein β-amyloid (Aß) oligomers starts intracellular indicating early toxicity [46]. At Al3+ presence occurs accumulation of Al3+-β-amyloid hydrophobic aggregates, which evolve to polymer aggregates due to increase in the conformation of β sheet [47]. Aggregates have a higher permeability through the blood brain barrier, Aß intracerebral accumulation happens. It had been showed Aß-Al3+ complexes display more aggregation than Aß protein [48]. Aluminum β-amyloid linkage causes a conformational change, increasing resistance to cathepsin D degradation, inhibiting neuritic plaque proteolysis, due to steric interference of Aß-Al3 +peptide [26,49]. Aß is derived from precursor proteins; these ones contain a homologous part a high degree, to bovine pancreatic trypsin inhibitor (BPTI), which also inhibits α-chymotrypsin. At AD brain, Al3+ favors proteolytic process that generates β-amyloid precursor [31]. Due α-chymotrypsin-BPTI linkage is favored by Al(III) uM concentrations, its proteolytic capacity is decreased, increasing amyloid plaque deposit [47,50,51].
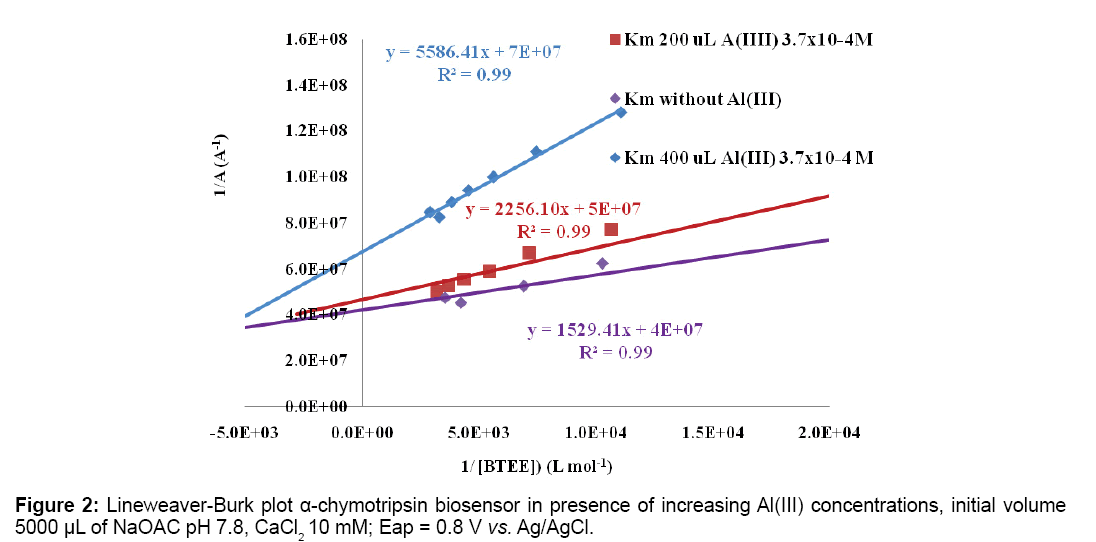
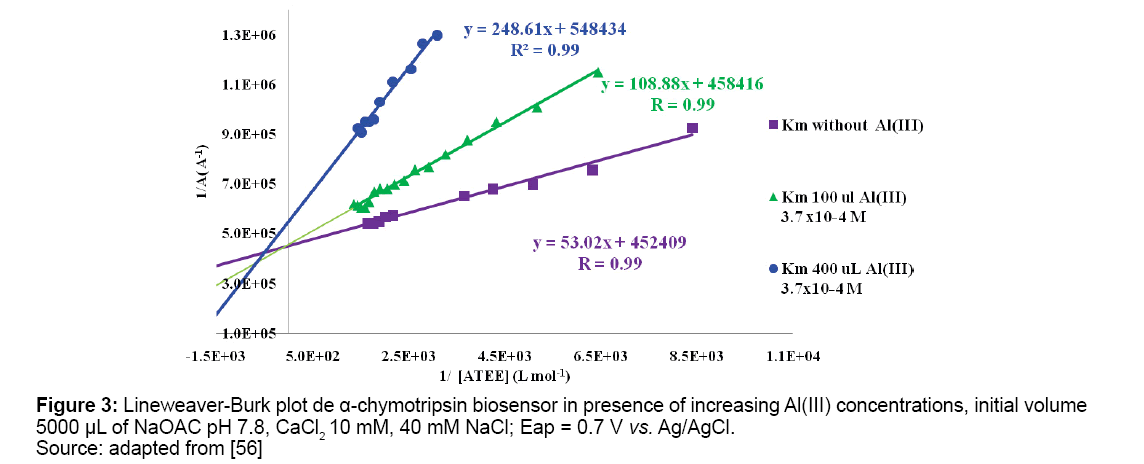
PHFs with NFTs are typical AD structures. PHFs are formed from abnormal τ protein isoforms of low molecular weight, there are found in microtubules, they are hyperphosphorylated at different sites. Phosphorylation results of a higher activity of kinase and lower of phosphatase. Al(III) ion induces a nonenzymatic catalysis of a covalent bond, incorporating phosphate ion of the nitrogenous bases in τ protein [33]. Phosphate linkage with Al(III) produced NFTs as irreversible process, larger τ protein oligomers are formed with Al3+ [52,53]. Aluminum administration to mice damages hippocampus and pyramidal cells lack of microtubules [54]. Low Al(III) concentration showed mixed inhibition on current oxidation of synthetic substrates benzoyl tyrosine ethyl ester (BTEE) and acetyl tyrosine ethyl ester (ATEE). Amperometric measurements were performed with screen printed carbon electrodes (SPCEs) with α-chymotrypsin immobilized on electrodes (Figures 2 and 3) [55,56].
Aluminum and Metal Ion Hypothesis
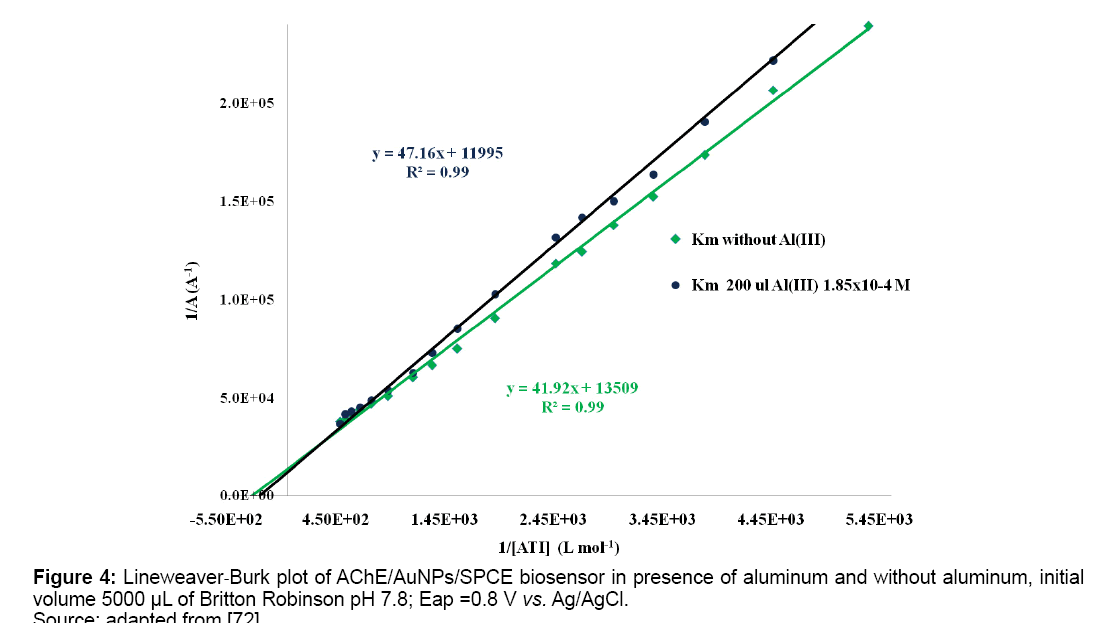
Impaired metal homeostasis occurs in AD; particularly Zn, Cu and Fe, with Aß imbalance resulting. Iron levels have been reported higher in AD neurons than neurons healthy individuals [57]. Transferrin transports iron and aluminum, but AD patients, transferrin carries more iron leaving more available aluminum in blood serum able to reach brain, ferritin too is higher in AD patients than in controls and complexes with aluminum, slowing iron uptake [58]. Amyloid precursor protein (APP) cascade is regulated and reacts with metal ions. There are two reservoirs of metal ions, the "free" (loosely bound) and capable to form chelates and the strongly bonded to protein or amino acid residues [59]. Zinc ion involved in amyloid cascade presents changes between its content in hippocampus of AD patients and cerebrospinal fluid of Parkinson's patients, suggesting that reserves free zinc/bound are changing constantly [36,60,61]. Endogenous metals as copper, iron, zinc and exogenous as aluminum may be involved as factors/cofactors in neurodegenerative processes [61]. Therapeutic strategies that restore metals homeostasis can delay development and modify the neurodegenerative processes associated with AD. This fact, strengths metal role in disease [62-64]. There are changes in endogenous homeostasis of Ca, Cu, Zn and Al metals in brain and liver tissue of laboratory animals. Al, Zn contents in hippocampus are higher and are lower in the cortex, but Cu and Ca decrease compared to controls [65]. Zn has been linked to enzymes involved in amyloid cascade and Cu is (SOD) enzyme cofactor [66]. Hippocampal cell cultures show different toxicity to different aluminum citrate complexes confirming transporters role [67]. AD patients were found to improve using Desferrioxamine (DFO), chelating agent of aluminum and iron, low intramuscular doses of DFO were used to reduce iron and aluminum excess in renal dialysis patients [68,69]. Health policies can take account existing information on the involvement of Al in AD to reduce its toxic effects [70]. The prevention and treatment of AD has focused in the use of antioxidants as resveratrol, saffron and curcuma, which counteract Al toxic effects and are used with anticholinergic drugs [39]. Aluminum content is higher in AD brains and patients show inhibition signs of ACE activity in cholinergic synapses. Response to aluminum injections in brain rats was degeneration of cholinergic terminals in cortex and hippocampus and inflammation. Therefore interference with cholinergic functions may be modes by aluminum may cause deficits of learning and memory and contributes to pathological processes in AD [71]. Aluminum inhibition on acetylcholinesterase was showed by mean of SPCEs using acetylthiocholine iodide (ATI), like Figure 4 where slope and also Km app values increase with Al (III) concentration [72].
Aluminum and Oxidative Stress Hypothesis
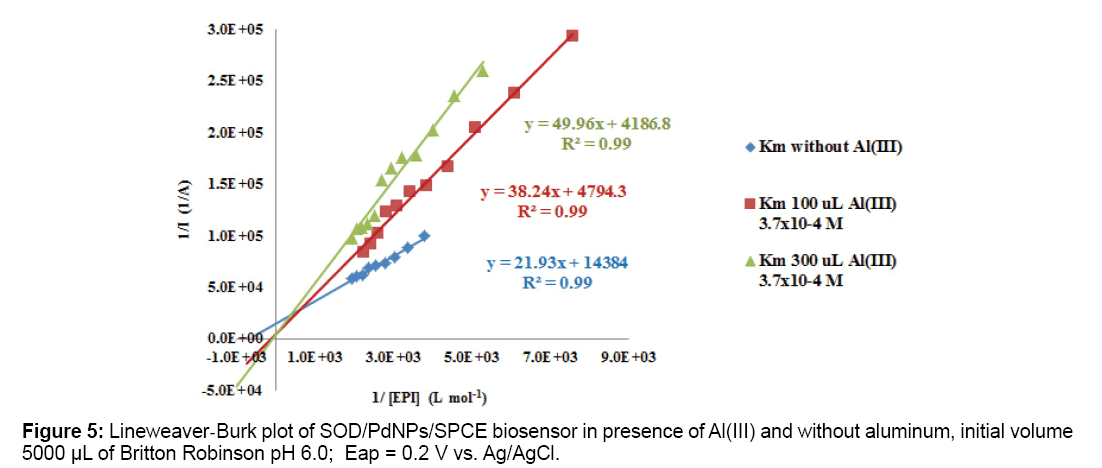
Age, environment and genetic factors accumulate defects on genes, then mitochondria functions decrease producing neurological disorders and neurons apoptosis starts. ROS and reactive nitrogen species (RNS) produce free radicals causing mutation and oxidative stress [73,74]. A defense against stress are SOD enzyme isoforms SOD-1 (intracellular) and SOD-3 (extracellular) which depend on Cu(I)/ Cu(II) and Zn(II) in their active sites respectively [75]. SOD mutations cause amyotrophic lateral sclerosis (ALS), that has similarities with AD, highlighting the importance of oxidative stress in neurodegenerative processes [76,77]. Metal ions involved in oxidative stress in AD are also involved in the appearance and disappearance of ROS and RNS in cell media [78,79]. Aβ-Al complexes aggregation produces mitochondrial dysfunction, because it increases activity of enzymes involved in the electron transport chain in mitochondria and ROS production. It also promotes neuronal cytoskeleton alterations, stimulating increased expression of enzymes that causes neurons conformational changes [80]. Aluminum can activate ROS generation and initiate AD inflammatory cascade producing oxidative stress and neurological damage [81]. There is possibility that Al3+ forms a stabilized complex AlO2 2+• -superoxide which reduces Fe3+ to Fe2+, which in turn is oxidized to Fe3+ to generate (OH•) radicals through Fenton reaction. Reaction between complex Al-superoxide and Fe3+ is spontaneous, loss of an electron is produced from the radical ion in AlO2 2+• -superoxide complex and molecular oxygen and Al3+ dissociates in a cyclical process. Other species such as Al(O2•)(H2O)5]2+, [Al(O2•)(OH)(H2O)4]+ and [Al(O2•) (OH)2(H2O)3], follow this same consideration. It is suggested that oxidizing activity of Al3+ in biological systems occurs through the mechanism of superoxide formation and recovering initial species of Al3+ and Fe3+ [82]. Interactions between aluminum and iron, which is initiator of oxidation processes have been studied in brain tissues by measuring ROS generation rates, combined use of Al2(SO4)3 y FeSO4 enhances the rate of appearance of ROS, ferritin presence which is linked to the bioavailability of mitochondrial iron produces Fe2+ excess, and oxygen presence creates free radicals that induce protein modification, lipid peroxidation and neuritic plaque formation [83,84]. Aluminum action on Deoxyribonucleic Acid (DNA) alters gene expression [85]. Sensitive enzymes to redox processes in Tricarboxylic Acid (TCA) cycle and oxidative phosphorylation are dramatically diminished by exposure to aluminum [86]. Aluminum is concentrated in brain regions affected in AD as the amygdala, cerebral cortex and hippocampus, where NFTs are found [87,88]. Aluminum phosphorylates proteins and alters their capacity to be transported along the axon, therefore Aß precursor accumulates within the axon and distends it [89]. Inhibition of Al (III) on SOD was tested on Epinephrine (EPI) substrate using palladium nanoparticles and superoxide dismutase enzyme immobilized on screen printed carbon electrodes modified with Tetrathiafulvalene (TTF), namely Pd/SOD/SPCTTFEs and competitive inhibition was obtained in presence of increasing Al (III) concentrations as it is shown in Figure 5. Slope of inverse of amperometric current vs inverse substrate concentration increases with Al(III) concentration accordingly with known enzymatic theory principles [90].
In Table 1 Km apparent inhibition values obtained for α-chymotrypsin, acetylcholinesterase and superoxide dismutase enzymes immobilized on SPCEs are shown. Al(III) exhibits its inhibitory action on tested enzymes at low concentrations.
| pH/ Potential/Al(III) Condition | SPCE Modification | Enzyme/Substrate | Kmapp Value |
|---|---|---|---|
| 7.8; 0.8 V Without Al Al(III) 1.42 × 10−5 M Al(III) 2.74 × 10−5 M | Gold NPs | α-chymotrypsin/ BTEE | [55] (3.6 ± 0.6) × 10-5 M (4.5 ± 0.6) × 10-5 M (8.3×± 0.5) 10-5 M. |
| 7.8; 0.7 V Without Al Al(III) 7.25 × 10−6 M Al(III) 2.74 × 10−5 M | Gold NPs | α-chymotrypsin/ ATEE | [56] (1.2× ± 0.1) 10-4 M (2.4× ± 0.1) 10-4 M (4.5× ± 0.3) 10-4 3 M |
| 7.8; 0.7V Without Al Al(III) 7.12 × 10−6M | Gold NPs | Acetylcholinesterase/ ATI | [72] (3.1 ± 0.3) × 10−3 M (3.9 ± 0.3) × 10−3 M |
| 5.0; 0.2V Without Al Al(III) 7.25 × 10−6 M Al(III) 2.18 × 10−5 M | Palladium NPs | Superoxide dismutase/ EPI | [90] (1.5 ± 0.3) × 10−3 M (5.0 ± 0.4) × 10−3 M (1.2 ± 0.3) × 10−2 M |
Table 1: Km apparent values obtained for tested enzymes with SPCEs.
Conclusion
AD is influenced by genetics, environmental factors, foods ingest and oxidative stress. It is difficult to judge if aluminum body burden found in AD is cause or consequence of disease. Role of aluminum on inhibited enzymes related with biological process that favors protein abnormal accumulation was demonstrated on α-chymotrypsin. Another important enzyme acetylcholinesterase related with nervous impulses transmission was inhibited in the presence of aluminum. Aluminum inhibition on SOD strengthens aluminum role in oxidative stress. At first screen printed electrodes modified with α-chymotripsin, acethyl cholinesterase and SOD were used to estimate Michaelis Menten inhibition constants. All tested enzymes were inhibited at very low aluminum concentration showing that aluminum enzymatic toxicity starts early and accumulates inside human body developing visible toxicity symptoms along life. Inhibitor effect is showed by increasing Kmapp values with Al (III) increasing concentration accordingly with enzymatic inhibition theory
Acknowledgement
Author acknowledges funding from the Vicepresidency for Research at the University of Costa Rica (Project 804-B5-117).
References
- Jansen S, Broadley MR, Robbecht E, et al. (2002). Aluminum hyper accumulation in angiosperms: A review of its phylogenetic significance. Bot Rev. 68: 235–269.
- Jansen S, Broadley MR, Robbecht E, et al. (2002). Aluminum hyper accumulation in angiosperms: A review of its phylogenetic significance. Bot Rev. 68: 235–269.
- Lowe ST. (1996). Enviromental hazards of aluminum to invertebrates, fish and wildlife. Rev Environ Contam Toxicol. 145: 1-127
- Kochian LV, Jones JL. (1997). Aluminum toxicity and resistance in plants. In: Yokel RA and Golub MS, (Eds), Research issues in aluminum toxicity, Taylor and Francis, Washington DC. 66–89.
- Polak TB, Milaci R, Pihlar B, et al. (2001). The uptake and speciation of various Al species in the Brassica rapapekinensis. Phytochemistry. 57: 198–198.
- Kochian LV, Jones JL. (1997). Aluminum toxicity and resistance in plants. In: Yokel RA and Golub MS, (Eds), Research issues in aluminum toxicity, Taylor and Francis, Washington DC. 66–89.
- Mozor-PietraszewskaT. (2001). Effect of aluminum in plant growth and metabolism. Acta Biochem Pol. 48: 673–686.
- Hodson MJ, Sangster AG. (1999). Aluminum/silicon interactions in conifers. J Inorg Biochem. 76: 89–98.
- Han J, Dunn MA. (2000). Effect of dietary aluminum on tissue non-heme and ferritin levels in the chick. Toxicology. 142: 97–109.
- Lima JC, Arenhart RA, Margis-Pinheiro M, et al. (2011). Aluminum triggers broad changes in microRNA expression in rice roots. Genet Mol Res. 10: 2817–2832.
- Golub MS, Han B, Kleen CL. (
- Han J, Dunn MA. (2000). Effect of dietary aluminum on tissue non-heme and ferritin levels in the chick. Toxicology. 142: 97–109.
- Metcalf PJ, Day JP, Garstang FM, et al. (1983). The determination of aluminum in bone. In: S.S Brown, J. Savory, (Eds), Chemical Toxicology and Clinical Chemistry of Metals. Academic Press, London. 53–56.
- Nieboer E, Gibson JL, Oxman AD, et al. (1995). Health effects of Aluminum: A critical review with emphasis on aluminum in drinking water. Environ Rev. 3: 29–81.
- Yokel RA, McNamara P. (2001). Aluminum toxico kinetics: An updated mini-review. Pharmacol Toxicol. 88: 159–167.
- Syracuse Research Corporation. (1999). Toxicologic profile for aluminum. Agency for Toxic Substances and Disease Registry, Public Health Services, Department of Health and Human Services, USDA.
- Metcalf PJ, Day JP, Garstang FM, et al. (1983). The determination of aluminum in bone. In: S.S Brown, J. Savory, (Eds), Chemical Toxicology and Clinical Chemistry of Metals. Academic Press, London. 53–56.
- Allen DD, Orving C, Yokel RA. (1995). Evidence for energy–dependent transport of aluminum out of brain extracellular fluid. Toxicology. 98: 31–39.
- Yokel RA. (2000). The toxicology of aluminum in the brain: A review. Neurotoxicology. 21: 813–828
- Flarend R, Bin T, Elmore D, et al. (2001). A preliminary study of the dermal absorption of aluminum from antiperspirants using aluminum-26. Food Chem Toxicol. 39:163–168.
- Akley DC, Yokel RA. (1997). Aluminum citrate is transported from brain into blood via monocarboxilic acid transporter located at the blood–brain barrier. Toxicology. 120: 89–97.
- Day JP, Barker J, Evans LJA, et al. (1991). Aluminum absorption studied by 26Al tracer. Lancet. 337: 1345–1345.
- Berthon G. (1996). Chemical speciation studies in relation to aluminum metabolism and toxicity. Coordin Chem Rev. 149: 241–280.
- Allen DD, Orving C, Yokel RA. (1995). Evidence for energy–dependent transport of aluminum out of brain extracellular fluid. Toxicology. 98: 31–39.
- Taylor GA, Ferrier IN, McLoughlin M, et al. (1992). Gastrointestinal absorption of aluminum in Alzheimer's disease: Response to aluminum citrate. Age Ageing. 21: 81–90.
- Cunat L, Lanhers MC, Joyeux M, et al. (2000). Bioavailability and intestinal absorption of aluminum in rats. Biol Trace Elem Res. 76: 31–55.
- Hem SL. (2002). Elimination of aluminum adjuvants. Vaccine. 20: 40–43.
- Akley DC, Yokel RA. (1997). Aluminum citrate is transported from brain into blood via monocarboxilic acid transporter located at the blood–brain barrier. Toxicology. 120: 89–97.
- Roskams AJ, Connor JR. (1990). Aluminum access to the brain: A role for transferrin and its detector. Proc Natl Acad Sci USA. 87: 9024–9027.
- Clauberg M, Joshi JG. (1993). Regulation of serine protease activity by aluminum: Implications for Alzheimer disease. Proc Natl Acad Sci USA. 90: 1009–1012.
- Crichton RR, Florence A, Ward RJ. (2002). Aluminum and iron in brain, prospects for chelation. Coordin Chem Rev. 228: 365–371.
- Zatta P. (1995). Aluminum binds to the hyper phosphorylated tau in Alzheimer’s disease. A hypothesis. Med Hypotheses. 44: 169–172.
- Alzheimer A. (1911). Ubereigenartige Krankheitsfälle des späteren Alters. Z Gesamte NeurolPsy.4: 356–385.
- Harrington CR, Wischik CM, McArthur FK, et al. (1994). Alzheimer's-disease like changes in tau protein processing: association with aluminium accumulation in brains of renal dialysis patients. Lancet. 343: 993–997.
- Taylor GA, Ferrier IN, McLoughlin M, et al. (1992). Gastrointestinal absorption of aluminum in Alzheimer's disease: Response to aluminum citrate. Age Ageing. 21: 81–90.
- Szutowicz A. (2002). Aluminum neurotoxicity. In: Massaro EJ. Handbook of Neurotoxicology. Humana Press Inc., Totowa, NJ, USA. 211–236.
- Zatta P, Kiss T, Suvalsky M, et al. (2002). Aluminum(III) as a promoter of cellular oxidation. Coordin Chem Rev. 228: 271–284.
- Kawhara M. (2005). Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. J Alzheimer’s Dis. 8: 171–182.
- Toda S, Yase Y. (1998). Effect of aluminum on iron-induced lipid peroxidation and protein oxidative modification of mouse brain homogenate. Biol Trace Elem Res. 61: 207–216
- Schliebs R, Arendt T. (2011). The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 221: 555–563.
- Clauberg M, Joshi JG. (1993). Regulation of serine protease activity by aluminum: Implications for Alzheimer disease. Proc Natl Acad Sci USA. 90: 1009–1012.
- Angeletti M, Lupidi G, Eleuteri AM, et al. (
- Zatta P. (1995). Aluminum binds to the hyper phosphorylated tau in Alzheimer’s disease. A hypothesis. Med Hypotheses. 44: 169–172.
- Abdel-Ghany M, El-Sebae AK, Halloway D. (1993). Aluminum-induced non-enzymatic phospho-incorporation into human tau and other proteins. J Biol Chem. 268:11976–11981.
- Harrington CR, Wischik CM, McArthur FK, et al. (1994). Alzheimer's-disease like changes in tau protein processing: association with aluminium accumulation in brains of renal dialysis patients. Lancet. 343: 993–997.
- Scott CW, Fieles A, Sygowski LA, et al. (1993). Aggregation of tau protein by aluminum. Brain Res. 628: 77–84.
- Bilkei-Gorzo A. (1993). Neurotoxic effect of enteral aluminium. Food ChemToxicol. 31: 357
- Meiri H, Banin E, Roll M, et al. (1993). Toxic effects of aluminum on nerve cells and synaptic transmission. Neurobiol. 40: 89–121.
- Kawhara M. (2005). Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. J Alzheimer’s Dis. 8: 171–182.
- Falkous G, Harris JB, Mantle D. (1995). Effect of neurotoxic metal ions in vitro on proteolytic enzyme activities in human cerebral cortex. Clin Chim Acta. 238: 125–135.
- Lupidi G, Angeletti M, Eleuteri AM, et al. (2002). Aluminum Modulation of proteolytic activities. Coordin Chem Rev. 228: 263–269.
- Kepp KP. (2012). Bioinorganic chemistry of alzheimer disease. Chem Rev
- Zatta P, Bordin C, Favarato M. (1993). The inhibition of trypsin and
- Paik SR, Lee JH, Kim DH, et al. (1997). Aluminum-induced structural alterations of the precursor of the non-
- Shin RW, Lee VM, Trojanowski JQ. (1994). Aluminum modifies the properties of Alzheimer's disease PHF tau proteins in vivo and in vitro. J Neurosci.14: 7221–7233.
- Barquero-Quirós M, Domínguez-Renedo O, Alonso- Lomillo MA, et al. (2015). Biosensor for aluminum (III) based on α-chymotrypsin inhibition using a disposable screen-printed carbon electrode and acetyl-tyrosine ethyl ester as substrate. Chemical Sciences Journal. 6: 2–6.
- Di Noto V, Dalla Via L, Zatta P. (2002). Review of binding methods and detection of Al(III) binding events in trypsin and DL-DPPC liposomes by a general thermodynamic model. Coordin Chem Rev. 228: 343–363
- Fleming J, Joshi JC. (1991). Ferritin: The role of aluminum in ferritin function. Neurobiol Aging. 12: 413–418.
- Zatta P, Suwalsky M. (2001). Aluminium, membranes and Alzheimer’s disease. In: C. Exley (Ed.), Aluminium and Alzheimer’s disease. The Science that Describes the Link.
- Banasik A, Lankoff A, Piskulak A, et al. (2005).
- Walsh DM, Tseng BP, Rydel RE, et al. (2000). The oligomerization of amyloid β protein begins intracellularly in cells derived from human brain. Biochemistry. 39: 10831–10839.
- Ricchelli F, Drago D, Filippi B, et al. (2005). Aluminum-triggered structural modifications and aggregation of β-amyloids. Cel Mol Life Sci. 62: 1724–1733.
- Banks WA, Niehoff ML, Drago D, et al. (2006). Aluminum complexing enhances amyloid β protein penetration of blood–brain barrier. Brain Res. 1116: 215–221.
- Sakamoto T, Saito H, Ishii K, et al. (2006). Aluminum inhibits proteolytic degradation of amyloid β peptide by cathepsin D: A potential link between aluminum accumulation and neuritic plaque deposition. FEBS Letters. 580: 6543–6549.
- Falkous G, Harris JB, Mantle D. (1995). Effect of neurotoxic metal ions in vitro on proteolytic enzyme activities in human cerebral cortex. Clin Chim Acta. 238: 125–135.
- Wiedau-Pazoz M, Goto J, Rabizadeh H, et al. (1996). Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 271: 515–518.
- Platt B, Drysdale AJ, Nday C, et al. (2007). Differential toxicity of novel aluminium compounds in hippocampal culture. Neurotoxicology. 28: 576–586.
- Lupidi G, Angeletti M, Eleuteri AM, et al. (2002). Aluminum Modulation of proteolytic activities. Coordin Chem Rev. 228: 263–269.
- Li W, Ma KY, Sun W, et al. (1998). Phosphorylation sensitizes microtubule-associated protein
- Bader B, Nübling G, Mehle A, et al. (2011). Single particle analysis of tau oligomer formation induced by metal ions and organic solvents. Biochem Biophys Res Commun. 411: 190–196.
- Walton JR. (2009). Brain lesions comprised of aluminum-rich cells that lack microtubules maybe associated with the cognitive deficit of Alzheimer’s disease. Neurotoxicology. 30: 1059–1069.
- Barquero-Quirós M, Domínguez-Renedo O, Alonso-Lomillo MA,
- Barquero-Quirós M, Domínguez-Renedo O, Alonso-Lomillo MA,
- Lovell MA, Robertson JD, Teesdale WJ, et al. (1998). Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci. 158: 47–52.
- Fleming J, Joshi JC. (1991). Ferritin: The role of aluminum in ferritin function. Neurobiol Aging. 12: 413–418.
- Xiao Z, Wedd A. (2010). The challenges of determining metal
- Rulon LL, Robertson JD, Lovell MA, et al. (2000). Serum zinc levels and Alzheirmer’s disease. Biol Trace Elem Res. 75: 79–85.
- Jimenez-Jimenez FJ, Molina JA, Aguilar MV, et al. (
- Freinbichler W, Colivicchi MA, Stefanini C, et al. (2011). Highly reactive oxygen species: Detection, formation, and possible functions. Cell Mol Life Sci. 68: 2067–2079.
- Bolognin S, Zatta P, Lorenzetto E, et al. (2013). β-Amyloid-aluminum complex alters cytoskeletal stability and increases ROS production in cortical neurons. Neurochem Int. 62: 566–574.
- Zatta P, Drago D, Bolognin S, et al. (2009). Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol Sci. 30: 346–353.
- Kawahara M. (2005). Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. J Alzeheimer Dis. 8: 171–182.
- Bondy SC, Kirstein S. (1996). The promotion of ironinduced generation of reactive oxygen species in nerve tissue by aluminum. Mol Chem Neuropathol. 27: 185–194.
- Schrag M, Mueller C, Oyoyo U, et al. (2011). Iron, zinc and copper in the Alzheimer's disease brain: A quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog Neurobiol. 94: 296–306.
- Exley C. (2012). Review. The coordination chemistry of aluminium in neurodegenerative disease. Coordin Chem Rev. 256: 2142–2146.
- Yang MS, Wong HF, Yung KL. (1998). Determination of endogenous trace metal contents in various mouse brains after prolonged oral administration of aluminum chloride. J Toxicol Env Health. 55: 445–453.
- Wiedau-Pazoz M, Goto J, Rabizadeh H, et al. (1996). Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 271: 515–518.
- Platt B, Drysdale AJ, Nday C, et al. (2007). Differential toxicity of novel aluminium compounds in hippocampal culture. Neurotoxicology. 28: 576–586.
- Shigematsu K, McGeer PL. (1992). Accumulation of amyloid precursor protein in damaged neuronal processes and microglia following intracerebral administration of aluminum salts. Brain Res. 593: 117–123.
- McLachlan CDR, Dalton AJ, KruckTPA, et al. (1991). Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet. 337: 1304–1308

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences