Adaptative Response of Chromohalobacter salexigens DSM 3043 to Saturated and Aromatic Hydrocarbons
Mihaela Marilena Lzroaie
Mihaela Marilena LÃÆââ¬Å¾ÃâÃâzÃÆââ¬Å¾ÃâÃâroaie*
Center of Microbiology,Institute of Biology,Romanian Academy,296 Spl. Independentei St,060031,PO 56-53,Bucharest,Romania
Abstract
Although it is known that Chromohalobacter salexigens DSM 3043 has the necessary genetic information to achieve the degradation of aromatic hydrocarbons, until now there is a lack of studies concerning the adaptative response of C. salexigens DSM 3043 to saturated and aromatic hydrocarbons. C. salexigens DSM 3043 was able to tolerate 1%, 5%, 10% (v/v) saturated monoaromatic and polyaromatic hydrocarbons. Monoaromatic hydrocarbons, characterized by a logarithm of the partition coefficient of the hydrocarbon in a mixture of octanol and water (log POW) between 2.14 and 3.14, were more toxic for C. salexigens DSM 3043 cells, if compared with polyaromatic and saturated hydrocarbons with log POW between 3.31 and 8.62. C. salexigens DSM 3043 was able to use as single source of carbon 1%, 5%, 10% (v/v) saturated or polyaromatic hydrocarbons and 1% (v/v) monoaromatic hydrocarbons (except benzene). A high sensitivity of this bacterial strain to the presence of 1% (v/v) benzene was observed. Saturated, polyaromatic and in particular monoaromatic hydrocarbons induced both cellular (cell viability, cell hydrophobicity) and molecular (protein profile, DNA) modifications in C. salexigens DSM 3043. The modifications induced by hydrocarbons in cells of C. salexigens DSM 3043 differ according to the nature of the hydrophobic substrate, its concentration and culture conditions.
Keywords
Chromohalobacter salexigens DSM 3043,hydrocarbons,adaptative response.
1. Introduction
Petroleum hydrocarbons spills occur frequently and they are a major cause of marine environment pollution. Approximately 2 to 6 million tons of crude oil enters marine environments each year,mainly from anthropogenic sources,but also pollution from natural marine oil seepages,oil platform incidents and tanker accidents results in considerable input of petroleum hydrocarbons in the marine environment [1]. Many catastrophic oil spills from large tanker accidents have attracted public attention to the fate of petroleum hydrocarbons in marine environments. In response to this concern,research into the biodegradation of petroleum in natural environments has been intensified. The behavior of pollutants in the environment is influenced primarily by the nature and amount of the contaminant present and the interplay among chemical,geochemical,and biological factors [2]. Among biological factors,the structure and dynamics of the indigenous microbial communities are major characteristics influencing biodegradation [1,3-8]. The degradation of complex pollutant mixtures such as petroleum requires a combination of different bacterial taxa that,when functioning as a community,can degrade a broader spectrum of hydrocarbons than any single bacterial species alone [9]. Several studies of contaminated sites have shown that the impact of contamination on bacterial communities is also dependent on the previous pollution history [9-11]. Many marine bacteria that are capable of degrading petroleum hydrocarbons have been recently isolated from different sites all over the world. However,few of them seem to be important for petroleum biodegradation in natural marine environments.
Chromohalobacter salexigens DSM 3043,formerly Halomonas elongate DSM 3043 [12],is a moderately halophilic bacterium,isolated by Vreeland et al. [13] from a solar saltern on Bonaire,Netherlands Antilles. It is an aerobic chemoorganotroph bacterium that grows on a wide range of simple carbon compounds,at NaCl concentrations between 0.5 M and 4 M,with an optimum at 2-2.5 M [12,14]. O’Connor and Csonka [15] observed that the growth rate of C. salexigens DSM 3043 can be stimulated in media containing 0.3 M NaCl by a 0.7 M concentration of other salts,such as Na+,K+,Rb+,or NH4 +,Cl−,Br−,NO3 -,or SO4 2- ions. Osmoadaptation of C. salexigens DSM 3043 is mainly achieved by de novo synthesis of ectoine and hydroxyectoine [14,16,17],but also bacterium can accumulates osmoprotectants such as choline,choline-O-sulfate and glycine betaine [14,18,19]. A nearly complete genome sequence of C. salexigens DSM 3043 has been obtained last year by DOE Joint Genome Institute in Genome Project [20].
Although it is known that C. salexigens DSM 3043 has the necessary genetic information to achieve the degradation of aromatic hydrocarbons,until now there is a lack of studies concerning the adaptative response of C. salexigens DSM 3043 to saturated and aromatic hydrocarbons. The biotechnological interest for moderately halophilic bacteria makes C. salexigens DSM 3043 an ideal candidate for such studies. The cellular and molecular modifications induced by saturated (decane,dodecane,tetradecane,pentadecane),monoaromatic (benzene,toluene,xylene isomers) and polyaromatic (naphthalene,anthracene,phenanthrene) hydrocarbons to C. salexigens DSM 3043 have been investigated in this study.
2. Methods
2.1 Tolerance and degradative capacity of C. salexigens DSM 3043 to saturated (decane,dodecane,tetradecane,pentadecane),monoaromatic (benzene,toluene,xylene isomers) and polyaromatic (naphthalene,anthracene,phenanthrene) hydrocarbons. Bacterial cells in the exponential phase of growth (109 cells/ml) were cultivated on liquid Halomonas reach medium (Halo-R) [12] (control) or liquid Halomonas minimal medium (Halo-M) [15],added with 0.1% sodium succinate (control) and on the same media in the presence of 1%,5%,10% (v/v) hydrocarbons. Flasks were sealed and incubated 24,48 hours at 37°C on a rotary shaker (150-200 rpm). The growth of the bacterial strain was determined by spetrophotometric measurement of the optical density (OD660nm). As there were no significant differences between the OD660 values measured after 24 and 48 h incubation in the presence of hydrocarbons,the only results presented in this paper are the results determined after 24 hours.
2.2 Cellular and molecular modifications induced by saturated (decane,dodecane,tetradecane,pentadecane),monoaromatic (benzene,toluene,xylene isomers) and polyaromatic (naphthalene,anthracene,phenanthrene) hydrocarbons on C. salexigens DSM 3043. Bacterial cells in the exponential phase of growth were cultivated on liquid Halo-R or Halo-M media (control) and on the same media in the presence of 0.1%,1% (v/v) hydrocarbons. The modifications induced by hydrocarbons on cellular (cell viability,cell hydrophobicity) and molecular (protein profile,DNA) level were determined after 24 h incubation at 37°C on a rotary shaker (150-200 rpm).
2.2.1 Modifications induced by hydrocarbons to cells viability. Serial dilutions of culture liquid were spread on agar medium using the method of Ramos et al. [21] and the number of viable cells (CFU ml-1) was determined.
2.2.2 Modifications induced by hydrocarbons to cell wall hydrophobicity. Bacterial adhesion to hydrocarbons was determined using the method of Rosenberg et al. [22]. The bacterial adhesion to hydrocarbons was also studied on wet mount with the Carl-Zeiss optical microscope.
2.2.3 Modifications induced by hydrocarbons to protein profile. Membrane and periplasmic protein fractions were extracted with HE buffer (10 mM HEPES-NaOH,pH 7.6,10 mM EDTA,10 mM MgCl2) solved in Laemmli buffer and denaturated at 95°C,for 5 min. 30 μg of protein per lane were loaded onto a 18% (w/v) polyacrylamide gel [23]. Gels were stained with Coomassie brilliant blue and destained in ethanol-glacial acetic acid-water (4.5:1:4.5 v/v/v) mixture. Protein content was measured by the method of Bradford [24].
2.2.4 Modifications induced by hydrocarbons to DNA. The bacterial cells were lysed with TE buffer (10 mM Tris-HCl,1 mM EDTANa2) and the DNA was extracted with phenol-chloroform-isoamilic alcohol (25:24:1 v/v/v) mixture,precipitated with ethanol and resuspended in TE buffer. DNA content and purity was measured by the method of Sambrook et al. [23]. For PCR amplification,1 μl of template DNA was added to a final volume of 25 μl of reaction mixture containing: 5×GoTaq flexi buffer,MgCl2,dNTP mix,primers (upstream primer 5’- GCCGTGGATGACGTCGATCT-3',downstream primer 3’-TGTTGGACAAGGACGAGTTCCA-5’),GoTaq DNA polymerase (Promega). PCR was performed with a PTC-150 thermal cycler (MJ Research,Watertown,Mass.). The PCR program consisted in initial denaturation for 2 min at 95°C,followed by 30 cycles of 1 min at 95°C,1 min at 58°C,1 min at 72°C,and a final extension of 5 min at 72°C. After separation on 1% (w/v) TAE (Tris- Acetate-EDTA) agarose gel and ethidium bromide staining,the PCR fragments were visualized with ultraviolet.
Biochemical reagents. Merck (E. Merck,Darmstadt,Germany),Sigma-Aldrich (Saint- Quentin-Fallavier,France),Promega (Promega GmbH,Mannheim,Germany) and BioLabs (Ipswich,New England) reagents were used in this study.
3. Results and Discussion
3.1 Tolerance and degradative capacity of C. salexigens DSM 3043 to saturated,monoaromatic and polyaromatic hydrocarbons. Microbial cells’ sensibility to hydrocarbons depends on the logarithm of the partition coefficient of the hydrocarbon in a mixture of octanol and water (log POW). The hydrocarbons toxicity is generally in inverse correlation with log POW,thus hydrocarbons with log POW between 1.5 and 3.5 are very toxic for microorganisms [6,25,26]. Despite this toxicity,it has been well established that marine microorganisms can degrade a wide variety of contaminants including those present in oil,such as alkanes and aromatic hydrocarbons [11,26,27].
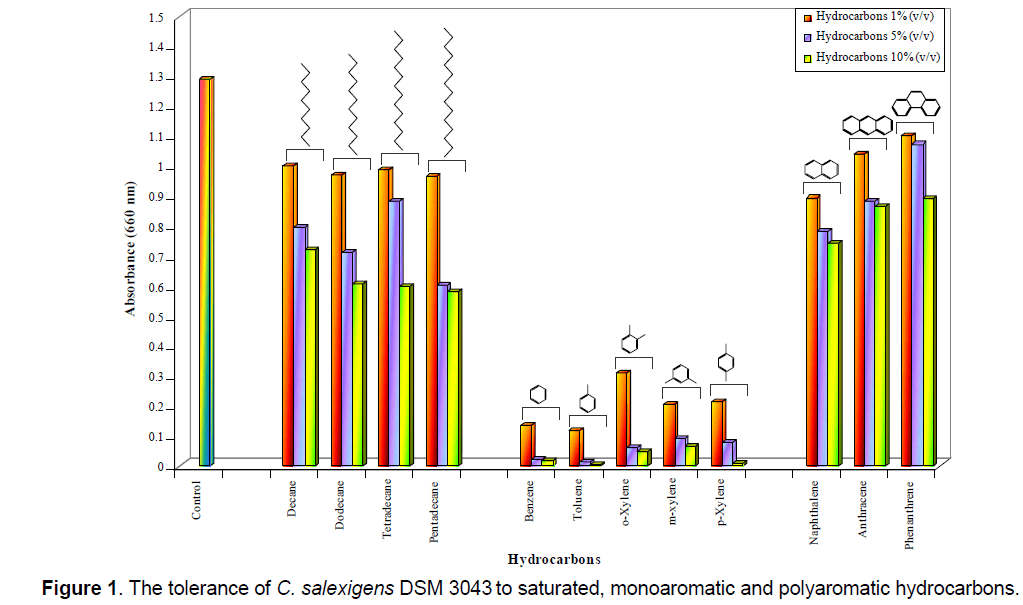
C. salexigens DSM 3043 presented high tolerance (OD660 = 0.784-1.170) to saturated (decane,dodecane,tetradecane,pentadecane) and polyaromatic (naphthalene,anthracene,phenanthrene) hydrocarbons used in concentrations of 1%,5% and 10% (v/v),but presented high sensitivity (OD660 = 0.009-0.089) to the presence in the liquid medium of the monoaromatic hydrocarbons (benzene,toluene,xylene isomers) in concentrations above or equal with 1% (v/v),except the xylene isomers in concentration of 1% (v/v),which were well tolerated (OD660 = 0.437-1.035) by the tested bacterial strain (Figure 1). The same results were obtained when it was tested the tolerance of C. salexigens DSM 3043 to saturated and aromatic hydrocarbons on solid medium (data not shown). It was observed an increased tolerance of C. salexigens DSM 3043 to saturated and polyaromatic hydrocarbons (CFU ml-1 were between 105 and 109) and an increased sensitivity of the bacterial cells to monoaromatic hydrocarbons (CFU ml-1 were 0).
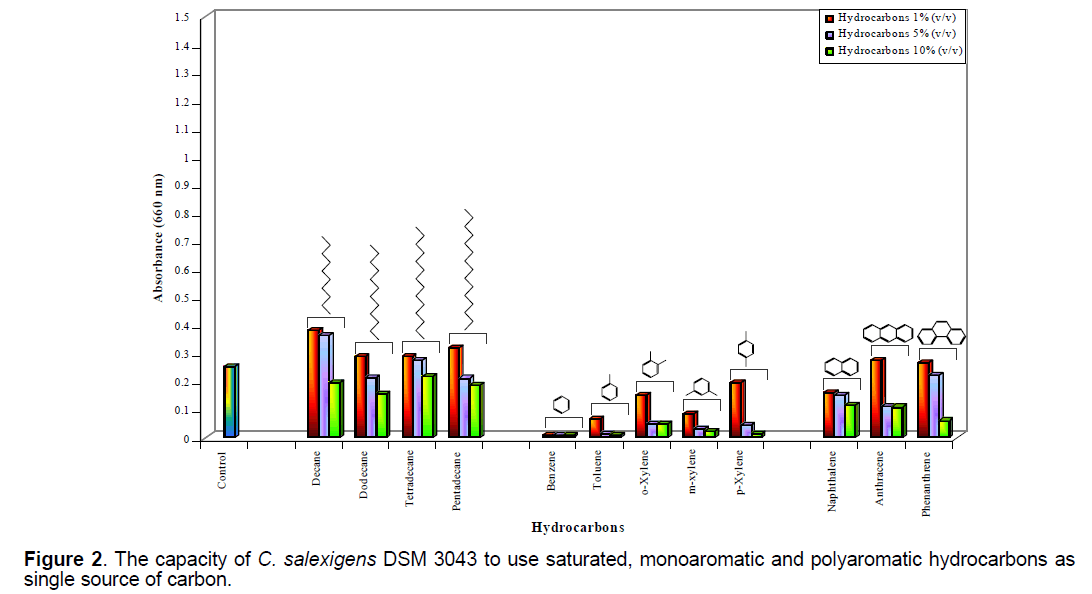
C. salexigens DSM 3043 was able to use (OD660 = 0.081-0.383) as single source of carbon saturated (decane,dodecane,tetradecane,pentadecane) and polyaromatic (naphthalene,anthracene,phenanthrene) hydrocarbons used in concentrations of 1%,5% and sometime 10% (v/v) (Figure 2 ).
C. salexigens DSM 3043 was able to use as single source of carbon (OD660 = 0.169-0.248) only some of the monoaromatic hydrocarbons (toluene,xylene isomers),used in concentration of 1% (v/v) (Figure 2 ). It was observed,like in previous assays,a high sensitivity of this bacterial strain to the presence of 1% (v/v) benzene in the culture medium.
The mechanism for hydrocarbons tolerance is still unknown,but some conclusions can be made from the current literature. The toxic effects of aromatic hydrocarbons were associated with loss of cytoplasmic membrane integrity. This loss of membrane integrity results in disruption of proton motive force,loss of membrane barrier functions,inhibition of membrane protein function,and subsequent cell lysis and death [6,25,28-32]. Hydrocarbons tolerance does not appear to be related to degradation,as most hydrocarbontolerant organisms tolerate a wide variety of aromatic hydrocarbons,alkanes,and alcohols,not degraded or transformed by them. Most of these organisms do not harbor plasmids,indicating that the resistance factors reside on the chromosome [6,25,30,31].
Therefore,when bacteria encounter a hazardous hydrocarbons,adaptive changes should occur in the structure of the cell membrane. For instance,alterations in membrane phospholipids [21,33,34],lipopolysaccharides [35],and membrane-embedded efflux pumps [6,36-38] have previously been reported to participate in increased hydrocarbon tolerance of bacteria.
Many bacterial adaptive responses to environmental changes are controlled by twocomponent signal transduction systems [32,39]. In a typical two-component system,an environmental stimulus is first detected by a transmembrane histidine sensor kinase,which is capable of autophosphorylation. The phosphoryl group is then transferred from a histidine to an aspartic acid residue in the second component of the pathway,a response regulator. Usually,the phosphorylated response regulator binds to the DNA,resulting in either activation or repression of target genes [40]. Two-component systems respond to a variety of environmental signals and regulate numerous functions,including cell division,sporulation,motility,metabolism,communication,virulence,stress adaptation,etc. There is also evidence of participation of two-component systems in the regulation of membrane permeability of bacteria [32,41].
3.2 Cellular and molecular modifications induced by saturated,monoaromatic and polyaromatic hydrocarbons on C. salexigens DSM 3043.
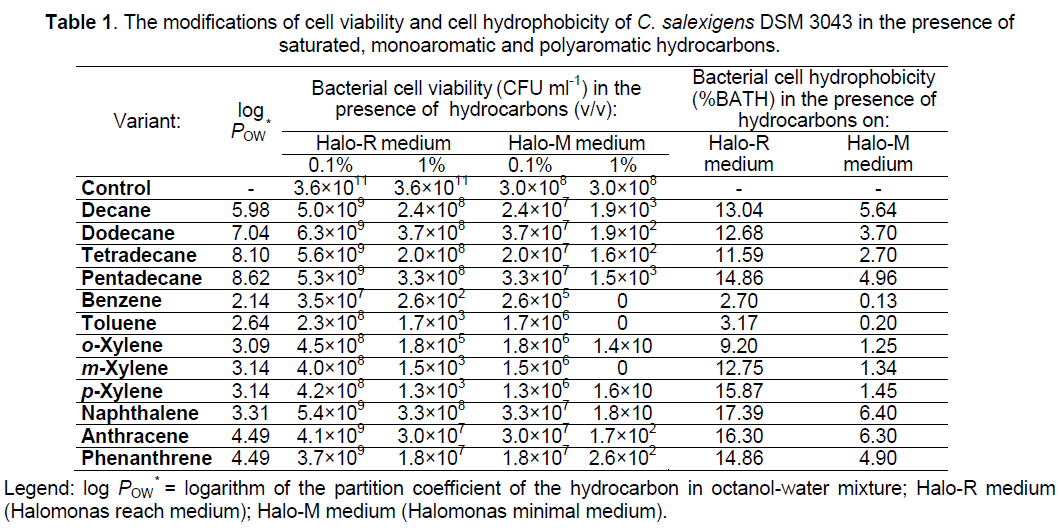
3.2.1 Modifications induced by hydrocarbons to cells viability (Table 1). The viability of C. salexigens DSM 3043 cells to saturated,monoaromatic and polyaromatic hydrocarbons differs according to the nature of the hydrophobic substrate,its concentration and culture conditions. The bacterial cells presented a higher viability (CFU ml-1 were between 102 and 109) when growth was done on reach medium,in the presence of the 0.1,1% (v/v) hydrocarbons,compared with the viability of the bacterial cells (CFU ml-1 were between 0 and 107) grown on minimal medium,in the presence of the same hydrocarbons. Saturated (decane,dodecane,tetradecane,pentadecane) and polyaromatic (naphthalene,anthracene,phenanthrene) hydrocarbons,with log POW between 3.31 and 8.62,were binding less abundantly to viable bacterial cells,being less toxic for them (CFU ml-1 were between 10 and 109),while monoaromatic hydrocarbons (benzene,toluene,xylene isomers),with log POW between 2.14 and 3.14,were binding more abundantly to viable bacterial cells,being more toxic for them (CFU ml-1 were between 0 and 108). The survival rates decreased significantly,below the detection limit of the experiment,when 1% (v/v) benzene,toluene and m-xylene were added to minimal medium.
Against the idea that the toxicity of a second phase of a hydrocarbon can be inferred from its hydrophobicity,reflected by its log POW [42],it was observed that some marine toluene-tolerant bacteria were unable to grow in the presence of some hydrocarbons with log POW values higher than toluene [26]. This was also previously observed for Pseudomonas putida DOT-T1E,which thrived in the presence of toluene (log POW = 2.64),but it was unable to do so in the presence of 1-octanol (log POW = 2.93). Although 1-octanol did not decrease cell viability,it completely impeded growth of the culture [43]. To explain these findings,the different partition of the hydrocarbon in the membrane was invoked [44]. However,the toxicity of a given hydrocarbon depends not only on its physicochemical properties but also on the specific response of the cells,and that this cellular response is not the same in all strains [26].
3.2.2 Modifications induced by hydrocarbons to cell wall hydrophobicity. The affinity of bacterial cells for hydrophobic interfaces is an important property that directly affects the efficiency of various bioprocesses,such as bioremediation and waste treatment,using whole microbial cells. Although the outcome of bacterial adhesion to hydrocarbon tests (BATH) is affected not only by hydrophobic interactions but also by van der Waals and electrostatic interactions [45],in this test method,the behavior of microbial cells in a two-liquid-phase system and the interaction of the cells with an organic phase,including the affinity of the cells for the organic surface,can be directly evaluated. Therefore,this method provides the best index when considering and designing systems in which bacterial conversion at the interface between the aqueous and organic phases is expected. These systems have received increasing attention for use in bioremediation and/or the treatment of oilcontaminated aqueous fields,as well as for microbial conversion in two-liquid-phase partitioning reactors [46].
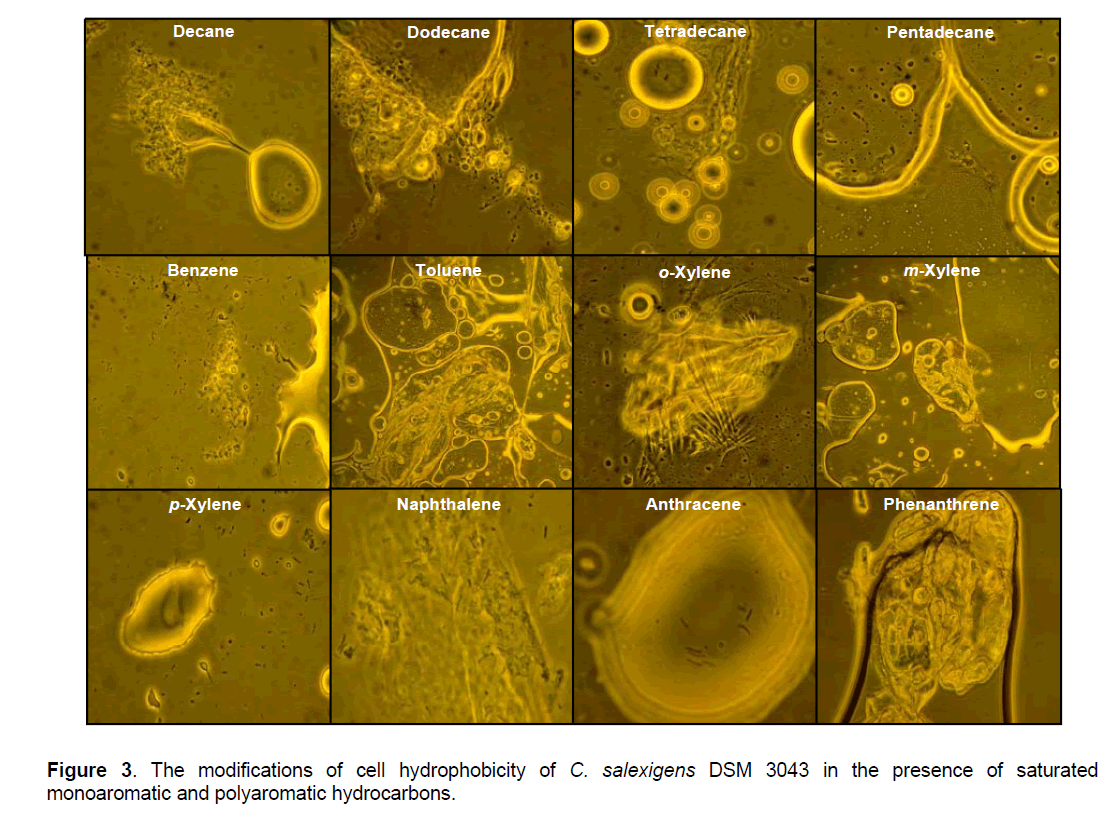
C. salexigens DSM 3043 cells presented higher hydrophobicity when the growth was done on reach medium (values between 2.7% and 17.39%),compared with cell hydrophobicity when the growth was done on minimal medium (values between 0.13% and 6.4%),fact confirmed by the optical microscope observations (Table 1,Figure 3 ).
Although the hydrocarbons are compounds with relatively low water solubility,the solubility rate may increase by increasing their specific surface,as a result of the mechanical dispersion realized by stirring the tubes containing the aqueous phase (cell suspension) and the organic phase (hydrocarbon) [47,48]. For saturated and monoaromatic hydrocarbons it was observed that the bacterial cells adhere to the microdroplets formed as a result of mechanical dispersion,which are stable,causing the decrease of the turbidity in the aqueous phase (values between 0.13% and 15.87%) (Table 1,Figure 3 ). For the polyaromatic hydrocarbons it was observed that the bacterial cells adhere to hydrocarbon crystals,causing also the decrease of the turbidity in the aqueous phase (values between 4.9% and 17.39%) (Table 1,Figure 3 ).
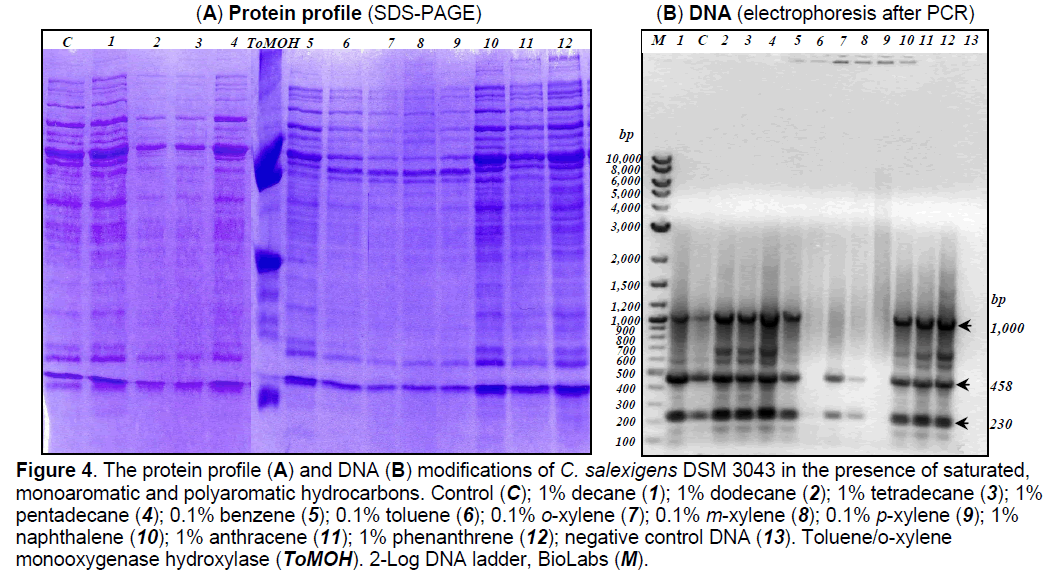
3.2.3 Modifications induced by hydrocarbons to protein profile. To investigate the modifications induced by hydrocarbons to membrane and periplasmic protein profile,one-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (1D SDS-PAGE) was used. Many more types of variation in protein sequences can be distinguished on one-dimensional gels in the absence of denaturants such as urea used in two-dimensional electrophoresis [49]. The electrophoresis studies showed the existence of some differences between membrane and periplasmic protein profiles extracted from C. salexigens DSM 3043 cells grown in the absence of hydrocarbons (control),compared with the protein profiles of the cells grown in the presence of saturated and aromatic hydrocarbons (Figure 4A). In the presence of 1% (v/v) decane,pentadecane,naphthalene,anthracene,phenanthrene it was observed the induction of the synthesis of some proteins,while in the presence of 1% (v/v) dodecane,tetradecane,0.1% (v/v) benzene,toluene,and xylene isomers it was observed the repression of proteins synthesis.
Figure 4. The protein profile (A) and DNA (B) modifications of C. salexigens DSM 3043 in the presence of saturated, monoaromatic and polyaromatic hydrocarbons. Control (C); 1% decane (1); 1% dodecane (2); 1% tetradecane (3); 1% pentadecane (4); 0.1% benzene (5); 0.1% toluene (6); 0.1% o-xylene (7); 0.1% m-xylene (8); 0.1% p-xylene (9); 1% naphthalene (10); 1% anthracene (11); 1% phenanthrene (12); negative control DNA (13). Toluene/o-xylene monooxygenase hydroxylase (ToMOH). 2-Log DNA ladder, BioLabs (M).
Recently,an extended proteomic survey was performed to identify all processes responsible for Pseudomonas putida DOT-T1E adaptation to toluene,showing that a whole cascade of mechanisms is necessary to allow the bacterium to survive in the presence of such toxic substance. The presence of toluene in the culture medium in which P. putida DOT-T1E has been cultured,provoked T drastic changes in the protein pattern. As part of this response,new proteins involved in hydrocarbon tolerance were synthesized and an increased expression of some preexisting proteins also took place,counteracting the decrease in activity due to membrane structural damage caused by the presence of toluene inside the cell [30].
The presence of high-molecular-weight polycyclic aromatic hydrocarbons (pyrene,pyrene- 4,5-quinone,phenanthrene,anthracene,fluoranthene) in the culture medium in which Mycobacterium vanbaalenii PYR-1 has been cultured provoked also changes in the protein pattern. Some proteins were detected uniquely upon exposure to a specific polycyclic aromatic hydrocarbon whereas others were common to more than one polycyclic aromatic hydrocarbon,which indicates that induction triggers not only specific responses but a common response in M. vanbaalenii PYR-1 [50].
3.2.4 Modifications induced by hydrocarbons to DNA. There were observed differences between the DNA extracted from C. salexigens DSM 3043 cells cultivated in the absence of the hydrocarbons (control) and those extracted from the cells cultivated in the presence of 1% (v/v) saturated or polyaromatic hydrocarbons and 0.1% monoaromatic hydrocarbons (Figure 4b).
Using as template the DNA extracted from C. salexigens DSM 3043 cells cultivated in the absence of the hydrocarbons and the DNA extracted from the cells cultivated in the presence of hydrocarbons,it was obtained the amplification of 458bp fragment,but also of another two fragments of 1000 bp and 230 bp (Figure 4B). It was observed a weaker amplification of the fragments when the used template was DNA extracted from the cells cultivated in the absence of hydrocarbons or in the presence of o-xylene and m-xylene,compared with the amplification of the same fragments when the used template was the DNA extracted from the cells cultivated in the presence of decane,dodecane,tetradecane,pentadecane,benzene,naphthalene,anthracene and phenanthrene. It was observed the lack of amplification of the fragments when the used template was the DNA extracted from the cells cultivated in the presence of toluene and p-xylene.
According with literature data [51,52] aromatic hydrocarbons are metabolically activated in cells to yield highly reactive bay region dihydrodiol epoxide derivatives. Dihydrodiol epoxides are electrophilic and can effectively attack DNA,forming covalently linked bulky adducts on DNA bases. These adducts cause structural changes in DNA,thus leading to disruption of normal cellular functions,such as transcription and replication. Furthermore,if not repaired,damaged nucleotides can result in mutations during replication.
Carney and Leary [53] observed that maintenance of phenotype in Pseudomonas putida R5-3 is achieved through a complex system involving an interaction between plasmid and chromosomal DNA. The particular aromatic hydrocarbon substrate utilized as the sole carbon source apparently induced the alterations in the biodegradative plasmids. Two indigenous plasmids,115 and 95 kilobases (kb) in size,were observed in R5-3A,which was derived from R5-3 by growth on minimal medium containing p-methylbenzoate as the sole carbon source. When R5-3A was transferred to medium containing m-xylene or toluene,derivative strains were obtained in which the 95-kb plasmid was lost and a new plasmid of 50 or 60 kb appeared. Reversion to the original plasmid profile of R5-3A was observed when xylene or toluene grown cells were returned to medium containing p-methylbenzoate. Restriction enzyme analysis and Southern blot hybridizations of total plasmid DNA indicated deletions and rearrangements of DNA restriction fragments in the derivatives maintained on m-xylene and toluene,when compared with the original R5-3A. Southern blot hybridizations revealed that part of the plasmid DNA lost from the original plasmid profile was integrated into the chromosomal DNA of xylene grown R5-3B and that these plasmid fragments were associated with aromatic hydrocarbon metabolism [53].
It was also attempted to induce the expression of some catabolic genes in C. salexigens DSM 3043 in the presence of 0.1% (v/v) saturated (dodecane),monoaromatic (benzene) and polyaromatic hydrocarbons (naphthalene). For RT-PCR amplification,5μl of RNA extracted with TRI Reagent (Sigma),were added to a final volume of 50μl of reaction mixture containing: 10×AMV/Tfl buffer,dNTP mix,primers (that were used in PCR reaction),MgSO4,AMV reverse transcriptase,Tfl DNA polymerase (Promega). The RT-PCR program consisted in reverse transcription at 45°C for 45 min,followed by initial denaturation for 2 min at 94°C,30 cycles of 1 min at 94°C,1 min at 60°C,1 min at 68°C,and a final extension of 7 min at 68°C. The unspecific amplification of a 150 bp fragment was obtained by RT-PCR,using as template the RNA extracted from bacterial cells cultivated on minimal medium added with 0.1% sodium succinate (control) and RNA extracted from cells cultivated on the same medium in the presence of 0.1% (v/v) dodecane,benzene and naphthalene (data not shown). Further studies will be carried out on this topic.
4. Conclusions
C. salexigens DSM 3043 was able to tolerate 1%,5%,10% (v/v) saturated (decane,dodecane,tetradecane,pentadecane),monoaromatic (benzene,toluene,xylene isomers) and polyaromatic (naphthalene,anthracene,phenanthrene) hydrocarbons. Monoaromatic hydrocarbons,characterized by a log POW between 2.14 and 3.14,were more toxic for C. salexigens DSM 3043 cells,if compared with polyaromatic and saturated hydrocarbons with log POW between 3.31 and 8.62. C. salexigens DSM 3043 was able to use as single source of carbon 1%,5%,10% (v/v) saturated or polyaromatic hydrocarbons and 1% (v/v) monoaromatic hydrocarbons (except benzene). A high sensitivity of this bacterial strain to the presence of 1% (v/v) benzene was observed. Saturated,polyaromatic and in particular monoaromatic hydrocarbons induced both cellular (cell viability,cell hydrophobicity) and molecular (protein profile,DNA) modifications in C. salexigens DSM 3043. The modifications induced by hydrocarbons in cells of C. salexigens DSM 3043 differ according to the nature of the hydrophobic substrate,its concentration and culture conditions. The study of cellular and molecular modifications induced by hydrocarbons in the moderately halophilic bacterium C. salexigens DSM 3043 showed a complex adaptative response of bacterial cells to the presence of different saturated and aromatic hydrocarbons in the culture medium. This study is relevant for better understanding the adaptation of bacteria inhabiting marine petroleumcontaminated environments to environmental fluctuations of hydrocarbon nutrients,for developing and implementing adequate bioremediation strategies of marine polluted environments.
Acknowledgements
This study was supported by a Short-Term EMBO Fellowship (ASTF no. 141-06),carried out in the Department of Structural and Functional Biology,University of Naples Federico II,Via Cinthia,80126 Napoli,Italy. I am especially indebted to Prof. Dr. Alberto Di Donato,Dr. Viviana Izzo,and Dr. Eugenio Notomista for providing me the Chromohalobacter salexigens DSM 3043 strain used in this study and also for the valuable discussions and for their help during the fellowship.
References
- Kalscheuer R.,Stöveken T.,Malkus U.,et al. (2007) Analysis of storage lipid accumulation in Alcanivorax borkumensis: evidence for alternative triacylglycerol biosynthesis routes in bacteria. J Bacteriol 189: 918- 928.
- Bordenave S.,Goñi-Urriza M.S.,Caumette P.,et al. (2007) Effects of heavy fuel oil on the bacterial community structure of a pristine microbial mat. Appl Environ Microbiol 73: 6089-6097.
- Harayama S.,Kishira H.,Kasai Y.,et al. (1999) Petroleum biodegradation in marine environments. J Mol Microbiol Biotechnol 1: 63-70.
- Syutsubo K.,Kishira H.,Harayama S. (2001) Development of specific oligonucleotide probes for the identification and in situ detection of hydrocarbondegrading Alcanivorax strains. Environ Microbiol 3: 371-379.
- Ramos J.L.,Duque E.,Gallegos M.T.,et al. (2002) Mechanisms of solvent tolerance in Gram-negative bacteria. Annu Rev Microbiol 56: 743-768.
- Hara A.,Syutsubo K.,Harayama S. (2003) Alcanivorax which prevails in oil-contaminated seawater exhibits broad substrate specificity for alkane degradation. Environ Microbiol 5: 746-753.
- Rhee S.K.,Liu X.,Wu L.,et al. (2004) Detection of genes involved in biodegradation and biotransformation in microbial communities by using 50-mer oligonucleotide microarrays. Appl Environ Microbiol 70: 4303-4317.
- Galvão T.C.,De Lorenzo V.,Mohn W.W. (2005) Exploring the microbial biodegradation and biotransformation gene pool. Trends Biotechnol 23: 497-506.
- Röling W.F.,Milner M.G.,Jones D.M.,et al. (2002) Robust hydrocarbon degradation and dynamics of bacterial communities during nutrient-enhanced oil spill bioremediation. Appl Environ Microbiol 68: 5537- 5548.
- MacNaughton S.J.,Stephen J.R.,Venosa A.D.,et al. (1999) Microbial population changes during bioremediation of an experimental oil spill. Appl Environ Microbiol 65: 3566-3574.
- Head I.M.,Jones D.M.,Röling W.F. (2006) Marine microorganisms make a meal of oil. Nat Rev Microbiol 4: 173-182.
- Arahal D.R.,García M.T.,Vargas C.,et al. (2001) Chromohalobacter salexigens sp. nov.,a moderately halophilic species that includes Halomonas elongata DSM 3043 and ATCC33174. Int J Syst Evol Microbiol 51: 1457-1462.
- Vreeland R.H.,Lichtfield C.D.,Martin E.L.,et al. (1980) Halomonas elongata,a new genus and species of extremely salt-tolerant bacteria. Int J Syst Bacteriol 30: 485-495.
- Cánovas D.,Vargas C.,Csonka L.N.,et al. (1996) Osmoprotectants in Halomonas elongata: high affinity betaine transport system and choline-betaine pathway. J Bacteriol 178: 7221-7226.
- O’Connor K.,Csonka L.N. (2003) The high salt requirement of the moderate halophile Chromohalobacter salexigens DSM 3043 can be met not only by NaCl but by other ions. Appl Environ Microbiol 69: 6334-6336.
- CalderÃÆÃÂÃâà ân M.I.,Vargas C.,Rojo F.,et al. (2004) Complex regulation of the synthesis of the compatible solute ectoine in the halophilic bacterium Chromohalobacter salexigens DSM 3043. Microbiol 150: 3051-3063.
- Vargas C.,Jebbar M.,Carrasco R.,et al. (2006) Ectoines as compatible solutes and carbon and energy sources for the halophilic bacterium Chromohalobacter salexigens. J Appl Microbiol 100: 98-107.
- Cánovas D.,Vargas C.,Iglesias-Guerra F.,et al. (1997) Isolation and characterization of salt-sensitive mutants of the moderate halophile Halomonas elongata and cloning of the ectoine synthesis genes. J Biol Chem 272: 25794-25801.
- Cánovas D.,Vargas C.,Csonka L.N.,et al. (1998) Synthesis of glycine betaine from exogenous choline in the moderately halophilic bacterium Halomonas elongata. Appl Environ Microbiol 64: 4095-4097.
- Galperin M.Y. (2006) Sampling of microbial diversity by complete genomes. Environ Microbiol 8: 1313-1317.
- Ramos J.L.,Duque E.,Rodriguez-Herva J.J.,et al. (1997) Mechanisms for solvent tolerance in bacteria. J Biol Chem 272: 3887-3890.
- Rosenberg M.,Gutnick D.,Rosenberg E. (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrofobicity. FEMS Microbiol Lett 9: 29-33.
- Sambrook J.,Fritsch E.F.,Maniatis T. (1989). Molecular Cloning,A Laboratory Manual,2nd ed. Cold Spring Harbor Laboratory Press,Cold Spring Harbor,New York.
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Sikkema J.,de Bont J.A.M.,Poolman B. (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59: 201-222.
- Segura A.,Hurtado A.,Rivera B.,et al. (2008) Isolation of new toluene-tolerant marine strains of bacteria and characterization of their solvent-tolerance properties. J Appl Microbiol 104: 1408-1416.
- Harayama S.,Kasai Y.,Hara A. (2004) Microbial communities in oil-contaminated seawater. Curr Opin Biotech 15: 205-214.
- Weber F.J.,Isken S.,de Bont J.A.M. (1994) Cis/trans isomerization of fatty acids as a defence mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology 140: 2013-2017.
- Segura A.,Duque E.,Mosqueda G.,et al. (1999) Multiple responses of Gram-negative bacteria to organic solvents. Environ Microbiol 1: 191-198.
- Segura A.,Godoy P.,van Dillewijn P.,et al. (2005) Proteomic analysis reveals the participation of energyand stress-related proteins in the response of Pseudomonas putida DOT-T1E to Toluene. J Bacteriol 187: 5937-5945.
- Van Hamme J.D.,Singh A.,Ward O.P. (2003) Recent advances in petroleum microbiology. Microbiol Mol Biol Rev 67: 503-549.
- Kivistik P.A.,Putrinš M.,Püvi K.,et al. (2006) The ColRS two-component system regulates membrane functions and protects Pseudomonas putida against phenol. J Bacteriol 188: 8109-8117.
- Pinkart H.C.,White D.C. (1997) Phospholipid biosynthesis and solvent tolerance in Pseudomonas putida strains. J Bacteriol 179: 4219-4226.
- Heipieper H.J.,Meinhardt F.,Segura A. (2003) The cis-trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: biochemistry,molecular biology and physiological function of a unique stress adaptive mechanism. FEMS Microbiol Lett 229: 1-7.
- Aono R.,Kobayashi H. (1997) Cell surface properties of organic solvent-tolerant mutants of Escherichia coli K-12. Appl Environ Microbiol 63: 3637-3642.
- Kieboom J.,Dennis J.J.,de Bont J.A.,et al. (1998) Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem 273: 85-91.
- Li X.Z.,Zhang L.,Poole K. (1998) Role of the multidrug efflux systems of Pseudomonas aeruginosa in organic solvent tolerance. J Bacteriol 180: 2987- 2991.
- Rojas A.,Duque E.,Mosqueda G.,et al. (2001) Three efflux pumps are required to provide efficient tolerance to toluene in Pseudomonas putida DOT-T1E. J Bacteriol 183: 3967-3973.
- Stock A.M.,Robinson V.L.,Goudreau P.N. (2000) Two-component signal transduction. Annu Rev Biochem 69: 183-215.
- Robinson V.L.,Buckler D.R.,Stock A.M. (2000) A tale of two components: a novel kinase and a regulatory switch. Nat Struct Biol 7: 626-633.
- Wang Y.,Ha U.,Zeng L.,Jin S. (2003) Regulation of membrane permeability by a two-component regulatory system in Pseudomonas aeruginosa. Antimicrob Agents Chemother 47: 95-101.
- Vermuë M.,Sikkema J.,Verheul A.,et al. (1993) Toxicity of homologous series of organic solvents for the Gram-positive bacteria Arthrobacter and Nocardia sp. and Gram-negative bacteria Acinetobacter and Pseudomonas sp. Biotechnol Bioeng,42: 747-758.
- Rojas A.,Duque E.,Schmid A.,et al. (2004) Biotransformation in double-phase systems: physiological responses of Pseudomonas putida DOTT1E to a double-phase made of aliphatic alcohol and biosynthesis of substituted catechols. App Environ Microbiol 70: 3637-3643.
- Neumann G.,Kabelitz N.,Zehnsdorf A.,et al. (2005) Prediction of the adaptability of Pseudomonas putida DOT-T1E to a second phase of a solvent for economically sound two-phase biotransformations. Appl Environ Microbiol 71: 6606-6612.
- Hori K.,Watanabe H.,Ishii S.,et al. (2008) Monolayer adsorption of a "bald" mutant of the highly adhesive and hydrophobic bacterium Acinetobacter sp. strain Tol 5 to a hydrocarbon surface. Appl Environ Microbiol 74: 2511-2517.
- Hori K.,Matsuzaki Y.,Tanji Y.,et al. (2002) Effect of dispersing oil phase on the biodegradability of a solid alkane dissolved in non-biodegradable oil. Appl Microbiol Biotechnol 59: 574-579.
- Rosenberg M. (1991) Basic and applied aspects of microbial adhesion at the hydrocarbon:water interface. Critical Reviews in Microbiology 18: 159-173.
- Heipieper H.J.,Weber F.J.,Sikkema J.,et al. (1994) Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol 12: 409-414.
- McLellan T.,Ames,G.F.-L.,Nikaido K. (1983) Genetic variation in proteins: comparison of onedimensional and two-dimensional gel electrophoresis. Genetics 104: 381-390.
- Kim S.-J.,Jones R. C.,Cha C.-J.,et al. (2004) Identification of proteins induced by polycyclic aromatic hydrocarbon in Mycobacterium vanbaalenii PYR-1 using two-dimensional polyacrylamide gel electrophoresis and de novo sequencing methods. Proteomics - Clinical Applications 4: 3899-3908.
- Wei S.-J.C.,Desai S.M.,Harvey R.G.,et al. (1984) Use of short DNA oligonucleotides for determination of DNA sequence modifications induced by benzoa]pyrene diol epoxide. Proc Nati Acad Sci USA 81: 5936-5940.
- Govindaswami M.,Feldhake D.J.,Kinkle B.K.,et al. (1995) Phylogenetic comparison of two polycyclic aromatic hydrocarbon-degrading mycobacteria. Appl Environ Microbiol 61: 3221-3226.
- Carney B.F.,Leary J.V. (1989) Novel alterations in plasmid DNA associated with aromatic hydrocarbon utilization by Pseudomonas putida R5-3. Appl Environ Microbiol 55: 1523-1530.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences